2029 Courses in St Neots
Get a 10% discount on your first order when you use this promo code at checkout: MAY24BAN3X
THIS COURSE PACKAGE INCLUDES: 1: INTRODUCTION TO PHLEBOTOMY COURSE (GPT003) - Level 3 (Ireland Level 5) 2: ADVANCED PHLEBOTOMY COURSE (GPT005) - Level 4 (Ireland Level 6) 3: GEOPACE COMPETENCY CERTIFICATE - CPD Certified (optional with Virtual Classroom) Learn how to take blood ... train as a Phlebotomist FAST-TRACK YOUR PHLEBOTOMY TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% off - Multi-Course Discount Cover all stages from beginner through to Level 4 Available as Classroom or Virtual Classroom Complete your beginner to advanced training in 2 days Awards 2 accredited qualifications - Introduction to Phlebotomy and Advanced Phlebotomy qualifications Both courses are dually accredited (OCN & CPD) Geopace Certificate of Competency included with classroom attendance or available as an option when booking virtual classroom Covers all steps up to live blood draw Learn advanced skills and techniques Virtual Classroom options include comprehensive Practise@Home Training Kits (yours to keep) Basic understanding of English language required OPEN TO ALL APPLICANTS
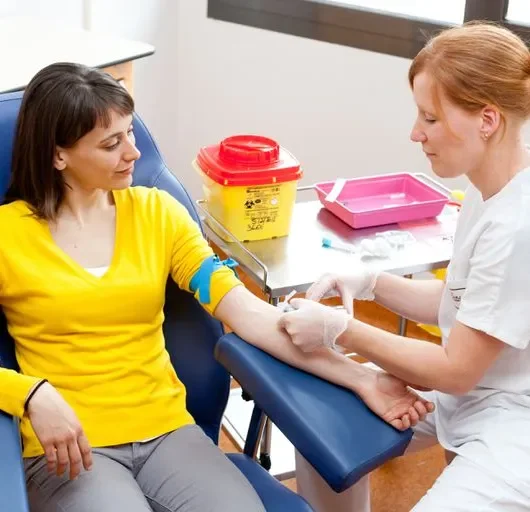
Learn how to take blood ... train as a Phlebotomist Nationally Recognised Qualification No previous experience or qualifications needed OCN Accredited - Level 3 (advanced) CPD Accredited (The CPD Certification Service) Covers all steps up to live blood draw Practise on artificial arm and fake blood! Basic understanding of English language required OPEN TO ALL APPLICANTS

The UK's first and only Level 4 qualification in Phlebotomy (equivalent to Ireland Level 6) FDSc (Foundation Degree Level) qualification Nationally Recognised certificate Dually accredited: Open College Network and CPD Covers both aspirated and evacuated systems Covers specialised blood collection systems & methods Classroom or Virtual Classroom learning options Comprehensive Training Kit is provided when booking our Virtual Classroom option (yours to keep) Complete your training from beginner to advanced level This course either follows on from our Introduction to Phlebotomy Course or can be combined with our introductory course as part of a course package (see below) Available to candidates who have completed (or are currently enrolled to complete) our Introduction to Phlebotomy Course or have previous phlebotomy practical experience.
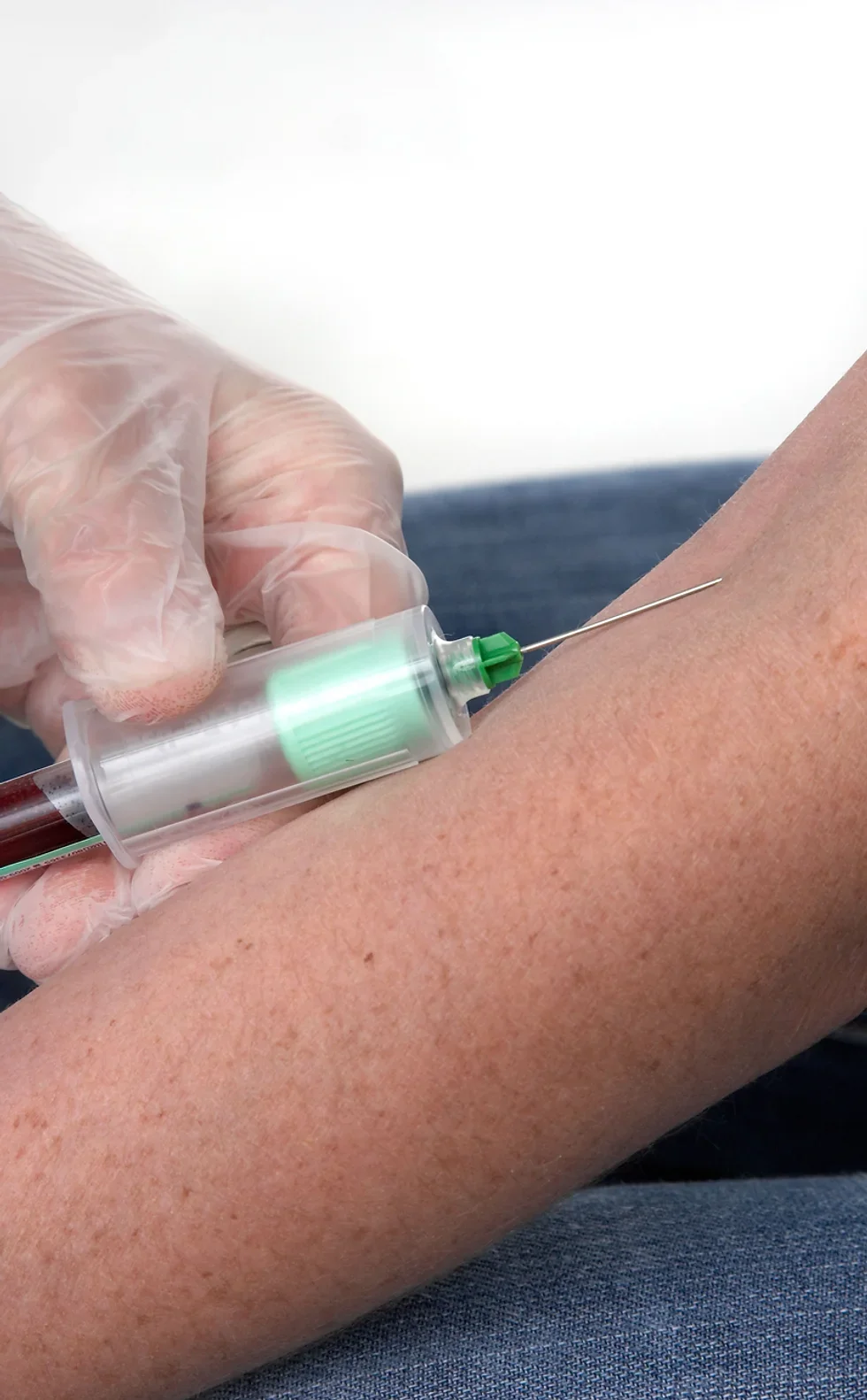
AVAILABLE ONLY TO APPLICANTS WHO HAVE ENROLLED ON (OR HAVE PREVIOUSLY COMPLETED) OUR INTRODUCTION TO PHLEBOTOMY COURSE Geopace Certificate of Competency Add live blood draws to your training Follow and complete a Competency Checklist Obtain tutor sign-off for live draws Awards a CPD Accredited Certificate Awards a Geopace Certificate of Competency Half day or evening sessions

St Neots Group Healing
By Carol Fenner
WHAT TYPE OF GROUP IS THIS? I provide healing to clients in a group, hosted in my studio in St Neots. These sessions were originally started in 2014, and ran for several years. I have decided to offer them again, as I feel that people need support in general. The healing rebalances the heart chakra, helps a person to feel grounded and safe, and releases tension and anxiety. These healing protocols are a small part of the full advanced frequency healing modality. Clients receive the healing seated, rather than laying flat on a couch. This helps me to see the areas that require attention. I have space for 4 clients in each session. This currently runs on a Sunday morning, starting at 11.00am and lasting approximately 60-90 minutes. It is important that you wear comfortable clothing, in layers to allow for the change of temperature in my studio. Please bring water to drink. I have toilet facilities and parking is unrestricted along my street, though I do ask that you respect my neighbours by parking safely and keeping noise to a minimum. For you to join I will require your full name, date of birth and email. This helps me to tune into your energy system and also send you any further information if necessary. All your personal information is completely confidential and never shared without your permission. You can book in advance and pay online, or pay cash on the day, though I will need to speak to you if you have not attended before. IMPORTANT INFORMATION * If you prefer to pay by cash, you can book by ringing me. Please bring the right amount with you and please book in at least 3 days before the day you wish to attend. * If you have booked by phone and are unable to attend, please let me know immediately so I can offer a space to someone else. It is very important that you let me know whether you can attend or not because it helps me to run my business effectively and means that you are acting in a consistent and honest way. * If you have booked online and cannot attend I will move you to the next session, or you can offer your slot to someone else, or I can run the session on you by distance.

Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
COURSE INFORMATION In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. WHO SHOULD ATTEND? * Auditors * Pharmacovigilance Quality System Managers * Pharmacovigilance scientists * The QPPV. COURSE BENEFITS Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: * A systematic investigation of the pharmacovigilance system and its quality system * Examination of how the pharmacovigilance system and quality system interact to achieve compliance. * The risk-based approach to auditing the PV system and quality system * The maintenance of 'inspection readiness' * Explore how to investigate the complex PV system * Discussions about how to monitor and maintain the PV system and assure compliance. COURSE OBJECTIVES CLARIFY WHAT HAS TO BE DONE: * Explore application of the legal requirements. EXPLORE HOW TO DO WHAT HAS TO BE DONE: * Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system * Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV * Explore how to investigate the complexity of the PV system. DISCUS HOW TO IDENTIFY WHAT IS MISSING OR WHAT NEEDS TO BE IMPROVED: * Discuss how to monitor and maintain the PV system and assure compliance. THIS COURSE WILL ASSIST DELEGATES WITH: * An understanding of key system principles, * A practical approach to implementing, maintaining and monitoring the PV system and its quality system * A procedure to share expertise to increase efficiency and confidence. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO: * Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. BY THE END OF THE COURSE DELEGATES WILL BE ABLE TO: * Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance * Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): JANA HYANKOVA Head of PV Department, IVIGEE Services a.s. -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:30 WELCOME, REGISTRATION, COURSE OBJECTIVES AND INTRODUCTION TO WORK GROUPS Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 THE REGULATORY FRAMEWORK FOR PHARMACOVIGILANCE Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 THE PHARMACOVIGILANCE SYSTEM Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 BREAK 11:00 WORKSHOP 1 AND FEEDBACK Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 THE QUALITY SYSTEM FOR PHARMACOVIGILANCE Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 LUNCH 14:00 WORKSHOP 2 AND FEEDBACK The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 THE QUALITY SYSTEM FOR PHARMACOVIGILANCE Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 DESCRIPTION OF PV SYSTEM 15:30 BREAK 15:30 WORKSHOP 3 AND FEEDBACK The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 THE PHARMACOVIGILANCE SAFETY MASTER FILE Construction of the Pharmacovigilance System Master File and its purpose. 17:00 WORKSHOP 3 AND FEEDBACK Description of PV System. 18:00 END OF DAY DAY 2 08:30 DRUG SAFETY IN THE CLINICAL TRIAL ENVIRONMENT - PART 1 Information flow and responsibilities of the sponsor. 09:30 WORKSHOP 4 AND FEEDBACK Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 BREAK 11:00 DRUG SAFETY IN THE CLINICAL TRIAL ENVIRONMENT - PART 2 Information flow and responsibilities of the sponsor. 12:00 LUNCH 13:00 WORKSHOP 5 AND FEEDBACK Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 PROCESSING OF SAFETY DATA Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 BREAK 15:30 EUDRAVIGILANCE Exploration of how EudraVigilance supports the PV system. 16:15 SIGNAL DETECTION AND EVALUATION/RISK BENEFIT ASSESSMENT: PHARMACOVIGILANCE RISK ASSESSMENT COMMITTEE (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 RISK MANAGEMENT PLANS A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 END OF DAY DAY 3 08:30 THE PHARMACOVIGILANCE RISK ASSESSMENT COMMITTEE (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 DEVELOPMENT SAFETY UPDATE REPORTS (DSURS): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 PERIODIC SAFETY UPDATE REPORTS (PSURS)/PERIODIC BENEFIT RISK EVALUATION REPORTS (PBRERS) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 BREAK 11:00 PERIODIC SAFETY UPDATE REPORTS (PSURS)/PERIODIC BENEFIT RISK EVALUATION REPORTS (PBRERS) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 WORKSHOP 6 AND FEEDBACK To explore the compilation and submission of the PSUR. 13:00 LUNCH 13:30 ROLE OF THE QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 BREAK 15:00 WORKSHOP 7 AND FEEDBACK To explore the challenges faced by the QPPV. 15:30 END OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-Face Course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device< * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD POINTS 23 Points DEVELOPMENT LEVEL Develop

Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
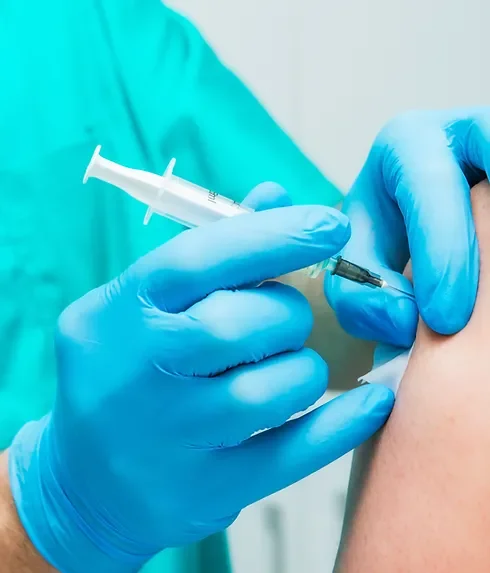
Platelet-rich Plasma (PRP) treatments Nationally Recognised Qualification No previous experience or qualifications needed Open College Network Accreditation Level 4 (as required for minimally invasive procedures) Covers standards set by HEE Employed (salon) or Self-Employed opportunities Basic understanding of English language required OPEN TO ALL APPLICANTS
Quality Assurance for Good Laboratory Practice
By Research Quality Association
COURSE INFORMATION A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. WHAT WILL I LEARN? * A solid regulatory foundation underpinning quality assurance activities * Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles * Enhanced efficacy in inspections and audits * Heightened compliance with Good Laboratory Practice standards for your facility * Unique insights into governmental monitoring activities within the GLP sphere. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO * Discuss and develop ideas * Solve specific problems * Examine particular aspects of GLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): CATE OVINGTON Director, The Knowlogy Group Ltd JANE ELLISTON Senior Quality Assurance Auditor, Battelle UK SHONA ROSS Head of QA, Tower Mains Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 WELCOME AND INTRODUCTIONS 09:15 GOOD LABORATORY PRACTICE STANDARDS AND REGULATIONS An insight into the background and history of Good Laboratory Practice. 09:45 PRINCIPLES OF QUALITY ASSURANCE What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 BREAK 10:45 STANDARD OPERATING PROCEDURES GLP requirements and QA involvement. 11:30 STUDY PLANS GLP requirements and QA involvement. 12:05 QA PROGRAMME Risk based programme, what are study, process and facility audits. 13:00 LUNCH 14:00 INSPECTIONS Attitudes, techniques and attributes. 14:40 WORKSHOP 1 - FACILITY AND PROCESS INSPECTIONS An exercise in inspection planning and preparation for inspections. 15:15 BREAK 15:30 WORKSHOP 1 - FEEDBACK 15:45 THE AUDITOR AND AUDIT CONDUCT Attitudes, attributes and techniques. 16:30 PANEL SESSION An opportunity for delegates to put questions to the panel of speakers. 17:15 CLOSE OF DAY DAY 2 09:00 WORKSHOP 2 - A MOCK AUDIT 10:45 BREAK 11:00 WORKSHOP 2 - FEEDBACK 11:30 AUDITING THE STUDY REPORT Techniques and methods for the QA audit of the study report. 12:00 RECORD KEEPING AND DATA The impact of GLP on data and records management. 12:40 LUNCH 13:25 DATA INTEGRITY A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 WORKSHOP 3 - AMENDMENTS TO STUDY PLAN AND DEVIATIONS FROM THE PLAN What are they? What is the difference between them? How are they controlled? 15:00 WORKSHOP 3 - FEEDBACK 15:15 BREAK 15:30 REGULATORY COMPLIANCE GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 PANEL SESSION An opportunity for delegates to put questions to the panel of speakers. 16:45 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Learn

Educators
Show all 1301Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham




