91 Computer Systems courses
Get a 10% discount on your first order when you use this promo code at checkout: MAY24BAN3X
Introduction to Computer Systems Validation
By Research Quality Association
COURSE INFORMATION Join our comprehensive three-day training programme tailored for individuals seeking a foundational grasp of computerised system validation. This course equips participants with essential knowledge to effectively validate systems within their respective organisations for utilisation in GxP (GLP, GCP, GMP, GDP, and GPvP) environments. Attendees will also gain proficiency in auditing validated computerised systems, ensuring compliance with pertinent GxP regulations. Commencing with an overview of regulatory prerequisites and the system life cycle, the course transitions into practical aspects, centered around validating computerised systems and conducting subsequent audits. Engage in a dynamic blend of presentations, interactive discussions, and hands-on practical workshops throughout the course. THIS COURSE WILL PROVIDE DELEGATES WITH AN UNDERSTANDING OF THE COMPUTERISED SYSTEM VALIDATION PROCESS, INCLUDING: * Definition of end user requirements * Risk management, including supplier assessment and techniques for audit planning * Validation planning and reporting * Linking system development with good business practices * Formal testing and qualification * Understanding of data integrity and security issues * How to assess system validation documentation to verify compliance. IS THIS COURSE FOR YOU? * IT professionals new to implementing computerised systems into regulated environments * Quality professionals who monitor or audit computerised systems * System owners, end users, tester and project staff. TUTORS Tutors will be comprised of (click the photos for biographies): NICHOLA STEVENS Director and Principal Consultant, Nuncius Compliance Solutions Ltd BARRY MCMANUS Consultancy Partner, Empowerment Quality Engineering Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 WELCOME, INTRODUCTION AND COURSE OBJECTIVES 09:45 WHY VALIDATE? REGULATIONS AND GUIDANCE ON COMPUTERISED SYSTEM VALIDATION Overview of the regulations and guidance applicable to CSV and their key expectations. 10:30 BREAK 10:45 THE SYSTEM LIFECYCLE The concept of the SLC and the key outputs from it. 12:00 LUNCH 12:45 THE VALIDATION PROCESS The approach to validation for different system types and a look at some of the key deliverables. 14:00 PROJECT INTRODUCTION 14:15 EXERCISE 1 - USER REQUIREMENTS Capturing, agreeing and documenting the user requirements for a system. 15:15 BREAK 15:30 EXERCISE 1 - FEEDBACK 16:00 RISK MANAGEMENT Risk management and its impact on validation. Identifying the deliverables required. Then group discussion on risk assessment for three systems. 17:00 QUESTIONS AND ANSWERS Answers to any outstanding questions from Day 1. 17:15 CLOSE OF DAY DAY 2 09:00 SUPPLIER ASSESSMENT The different approaches to supplier assessment and the things to be considered when assessing a supplier. 10:15 EXERCISE 2 - SUPPLIER ASSESSMENT Planning a vendor audit with a focus on the key validation deliverables. 11:00 BREAK 11:15 EXERCISE 2 - FEEDBACK 11:45 TEST OVERVIEW AND TEST PLANNING The different test phases, the purpose of each test phase and things to be considered when planning and reporting testing. 12:45 LUNCH 13:30 TEST OVERVIEW AND TEST PLANNING Continued. 14:15 TEST SCRIPT DESIGN, EXECUTION AND REVIEW What a good test script looks like and the key things to consider when creating, executing and reviewing a test script. 15:30 BREAK 15:45 EXERCISE 3 - CREATING A TEST SCRIPT Create a test script based on user requirements created on Day 1. 17:15 CLOSE OF DAY DAY 3 09:00 EXERCISE 3 FEEDBACK 09:30 INFRASTRUCTURE CONFIGURATION AND QUALIFICATION 10:30 BREAK 10:45 VALIDATION REPORTING Overview of the Validation Report and what should be included in it. 11:15 MAINTAINING THE VALIDATED STATE The procedures and records needed to ensure the system remains fit for purpose. 12:30 LUNCH 13:15 CHANGE CONTROL Key concepts related to making changes to validated systems. 14:00 DATA INTEGRITY AND SECURITY How can we assure the integrity and security of our data. 15:15 BREAK 15:30 COURSE OBJECTIVES SUMMARY AND PANEL DISCUSSION A round up of key learning from the course. 17:00 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 19 Points DEVELOPMENT LEVEL Develop

50255 Managing Windows Environments with Group Policy
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for This course is intended for Enterprise network staff responsible for help desk support, system administration, and network design. Overview At Course Completion: - Features and functions of Group Policy - Use Group Policy management tools to manage security policies - Design a Group Policy infrastructure - Group Policy processing architecture - Back up, restore, import, and copy Group Policy Objects through the Group Policy - Management Console - Use Windows PowerShell to manage Group Policy - Implement security using Group Policy - Configure the desktop environment with Group Policy - Configure roaming profiles and Folder Redirection - Assign and publish software packages - Implement AppLocker and software restriction policies - Create and deploy Administrative Templates - Configure Group Policy preferences In this course, you will learn how to reduce costs and increase efficiencies in your network. You will discover how to consolidate the administration of an enterprise IT infrastructure with Group Policy, and you will learn to control and manage computer systems and domain users running Windows Server 2016 and Windows clients. Create Group Policies, implement administrative and security templates, and determine best practices when deploying software packages. Walk away with the experience and tools needed to optimize your enterprise systems and networks, implement administrative and security templates, and determine best practices when deploying software packages. 1 - INTRODUCTION TO CONFIGURATION MANAGEMENT * Configuration Management Overview * Configuration Management Using Group Policy * Group Policy Features Introduced with Later Versions of Windows * Using Windows PowerShell in the Configuration Management Process 2 - USING GROUP POLICY MANAGEMENT TOOLS * Local and Domain Policies * Using Group Policy Management Console * Understanding Group Policy Refresh 3 - DESIGNING A GROUP POLICY INFRASTRUCTURE * Design Stages for a Group Policy Infrastructure * Planning your Group Policy Design * Designing Your Group Policy Solution * Deploying Your Group Policy Solution * Managing Your Group Policy Solution 4 - UNDERSTANDING THE ARCHITECTURE OF GROUP POLICY PROCESSING * Group Policy Components in AD DS * Understanding the Group Policy Deployment Order * Modifying Group Policy Processing 5 - TROUBLESHOOTING AND BACKING UP GPOS * Using Group Policy Troubleshooting Tools * Integration of RSoP Functionality * Group Policy Logging Options * Backing Up, Restoring, Importing, and Copying GPOs * Building Migration Tables 6 - SECURING WINDOWS USING GROUP POLICY * Exploring the Windows Security Architecture * Securing User Accounts * Exploring Security Policies * Windows Firewall with Advanced Security * Implementing Domain Security * Security Policy Options for Window Client and Windows Server 7 - IMPLEMENTING APPLICATION SECURITY USING GROUP POLICY * Managing UAC Settings * Defending Against Malware * Using AppLocker to Restrict Software * Implementing Windows App Security Settings 8 - CONFIGURING THE DESKTOP ENVIRONMENT WITH GROUP POLICY * Exploring Script Types and Controlling Script Execution * Defining the Desktop, Start Menu, and Taskbar Settings * Defining the Control Panel Settings * Defining the Windows Components Settings * Configuring the Printer Management and Pruning Settings * Defining the Network Settings * Discovering the new Group Policy Settings 9 - IMPLEMENTING USER STATE VIRTUALIZATION * Configuring Folder Redirection * Managing Offline Files * Implementing User Experience Virtualization * OneDrive for Business 10 - ASSIGNING AND PUBLISHING SOFTWARE PACKAGES * Using MSI Packages to Distribute Software * Using Group Policy as a Software Deployment Method * Deploying Software * Setting Up Distribution Points * Using the Systems Center Configuration Manager to Distribute Software 11 - CONFIGURATION MANAGEMENT WITH WINDOWS POWERSHELL * Introducing Windows PowerShell * Using Windows PowerShell * Writing PowerShell Scripts * Windows PowerShell Library for Group Policy * Windows PowerShell-Based Logon Scripts 12 - IMPLEMENTING POWERSHELL DESIRED STATE CONFIGURATION * Introducing Group Policy Preferences * Comparing Preferences and Policy Settings * Options for Applying Preferences * Configure Preference Settings 13 - CONFIGURING GROUP POLICY PREFERENCES * Introducing Group Policy Preferences * Comparing Preferences and Policy Settings * Options for Applying Preferences * Configuring Preferences Settings ADDITIONAL COURSE DETAILS: Nexus Humans 50255 Managing Windows Environments with Group Policy training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the 50255 Managing Windows Environments with Group Policy course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
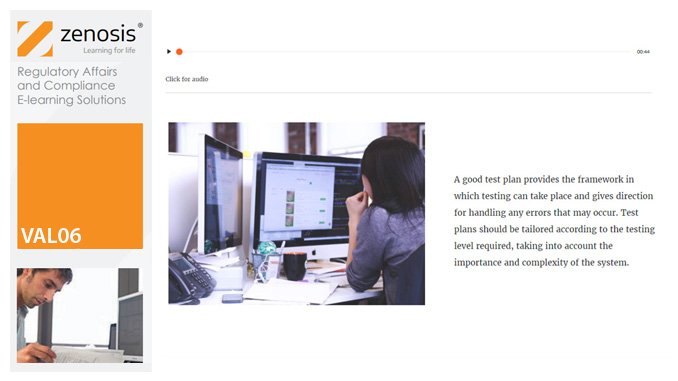
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.
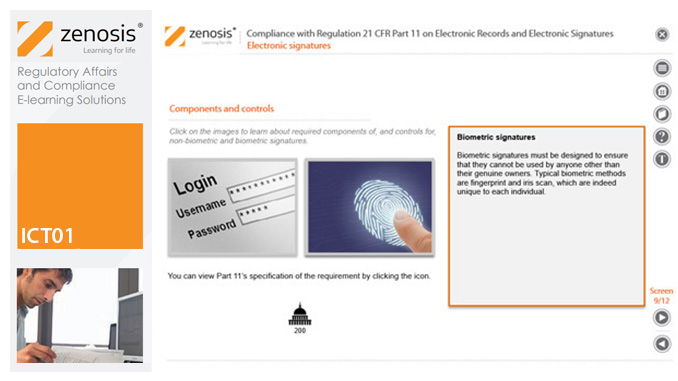
Quality Systems for Research Laboratories
By Research Quality Association
COURSE INFORMATION This highly interactive course will provide guidance on why and how to implement a quality system successfully into the research laboratory. By doing so, you will position your innovation for the success it deserves. But leave things as they are and there is a good chance that your science will not realise its full potential should success, and its consequences, come your way. A quality system in your research laboratory is the most effective and efficient way to: * Help scientists work more efficiently * Ensure discoveries can be defended * Protect the value of intellectual property. This course is particularly aimed at those working in early phase research environments which are not constrained by the regulatory requirements of the Good Practice regulations but are producing intellectual property, testing and/or products for the therapeutic market. For organisational reasons, rather than regulatory ones, this is a place where you need to get it right. The programme is delivered by leaders in the field who, quite simply, ‘have done it’. Whether delegates are at senior management level seeking strategic direction, a laboratory head wishing to deliver science that will stand the test of time or a quality professional thrown in at the deep end, this course will provide key insight and practical guidance to underpin future success. Based on risk based systems, tried and tested over many years in the workplace, the programme will help delegates to define, train, implement and monitor the quality of their research, irrespective of field or discipline. Delegates will learn how to help position their organisation for success. COURSE CONTENT: Delegates will be guided thoughtfully through each key component of the process in a stimulating learning environment. The course probes all avenues of the research quality arena, from an initial understanding of the cultural aspects of the scientific discovery environment, to managing quality in outsourced research programmes. Computer systems and e-data security in the research environment will be discussed and pragmatic solutions described to help manage the ballooning cloud of e-data. In addition, the ever blurring boundary between the regulated and non-regulated research environments will be discussed and delegates given perspective on future developments in the field. With this knowledge, delegates will be able to get it ‘right first time’. IS THIS COURSE FOR YOU? The course is designed for all those involved in the research laboratory quality arena and it has been tailored to meet the needs of scientific management, bench scientists and quality professionals alike. Delegates get immediate access to highly experienced tutors who will share their wisdom and insights in an area where few others have been successful. The course is linked with the RQA guidance which builds on years of experience and forms the foundation of the programme. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): LOUISE HANDY Director, Handy Consulting Ltd SANDRINE BONGIOVANNI Associate Director in Research and Quality Compliance, Novartis -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 REGISTRATION 09:10 WELCOME AND INTRODUCTIONS 09:20 HISTORY AND OVERVIEW OF THE FIELD Examples of business and regulatory risks and the consequences of low quality in research. A look at the standards and guidelines that exist. 10:00 THE CULTURE, THE POLITICS AND THE SCIENTIST'S PERSPECTIVE Understanding research environments, the drivers and the challenges. 10:30 BREAK 10:45 WORKSHOP - RISK MANAGEMENT Thinking about risk management and prioritisation. Looking at the critical factors for the implementations of a successful quality system. 12:15 WORKSHOP - FEEDBACK 12:45 LUNCH 13:45 PERSONNEL, PLANS, PROCEDURES, FACILITIES, EQUIPMENT, MATERIALS AND REAGENTS Looking at planning the work, defining procedures in a way which promotes robust science without compromising brilliance and ensuring that all these elements are demonstrably fit for their intended purpose. 14:30 WORKSHOP - ASSAY VALIDATION How much validation is required at what stage? What do we need to validate an assay? 15:00 WORKSHOP - FEEDBACK 15:15 RESEARCH, WORK RECORDS, ARCHIVES AND RESEARCH REVIEW Data and records which are accurate, attributable, legally attestable and safe to permit reconstruction experiments and studies. Looking at aspects of the work where there is a chance to review, correct or improve the science, the data and the processes. 16:15 CONTINUAL IMPROVEMENT AND QUALITY SYSTEMS Reviewing implementation of a quality system, finding opportunities for improvement, understanding culture change. 16:45 QUESTIONS AND ANSWERS 17:00 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 7 Points DEVELOPMENT LEVEL Develop

Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

CYBER SECURITY TRAINING COURSE DESCRIPTION The reliance of the world on the Internet and computer systems means the protection of information systems is vitally important. This is even more important smart devices and the Internet of Things increase the number of devices. This Cyber Security provides a concise overview on the threats and attacks that can happen along with the counter measures that can be taken. WHAT WILL YOU LEARN * Recognise the different types of attack. * Describe the attacks that can be made against information systems. * Describe the counter measures available. CYBER SECURITY TRAINING COURSE DETAILS * Who will benefit: Non-technical staff working with computers. * Prerequisites: None. * Duration 1 day CYBER SECURITY TRAINING COURSE CONTENTS * What is Cyber Security? Computer security, Policies, Types of security breach, denial of service, data manipulation, data theft, data destruction, security checklists, incident response. * Attacks Physical access, Social engineering, Privilege escalation, Malware, Trojans, worms, viruses, rootkits, Backdoors, Denial of Service (DOS), Distributed DOS, Eavesdropping, Spoofing, Man the middle tampering. * Countermeasures Prevention, detection, response. Physical, user accounts, Firewalls, IDS, AAA, authentication, cryptography, encryption, data integrity. * Cyber security standards ETSI, ISO 27001, 27002, NIST, ISA/IEC 62443.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
COURSE INFORMATION Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. IS THIS COURSE FOR YOU? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. THIS COURSE WILL GIVE YOU: * Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) * Insight into the seamless integration of GCLP within clinical programmes (GCP) * Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories * The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) * Access to a seasoned panel of speakers with extensive expertise * A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. ENGAGE IN: * Lively discussions to foster ideas * Problem-solving sessions targeting specific challenges * Detailed exploration of specific aspects within the realms of GCP and GCLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): VANESSA GRANT -, - LOUISE HANDY Director, Handy Consulting Ltd TIM STILES Consultant, Qualogy Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:50 REGISTRATION 09:00 WELCOME AND INTRODUCTION 09:20 GOOD CLINICAL PRACTICE AND THE REQUIREMENTS OF GOOD CLINICAL LABORATORY PRACTICE A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 SAFETY AND ETHICAL CONSIDERATION Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 BREAK 10:55 ORGANISATION AND PERSONNEL RESPONSIBILITIES WITHIN GCP AND THE LABORATORY The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 STAFF TRAINING AND TRAINING RECORDS Personnel records of training and competency assessments are discussed. 11:45 LABORATORY FACILITIES, EQUIPMENT AND MATERIALS Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 LUNCH 13:15 WORKSHOP 1 - FACILITIES, EQUIPMENT AND RESPONSIBILITIES Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 WORKSHOP 1 - FEEDBACK 14:15 COMPUTER SYSTEMS VALIDATION Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 TRIAL PROTOCOLS, ANALYTICAL PLANS During this session we examine the purpose, content, control and change of these important documents. 15:30 BREAK 15:45 WORKSHOP 2 - SOPS, CLINICAL PROTOCOLS, ANALYTICAL PLANS AND VALIDATION The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 WORKSHOP 2 - FEEDBACK 17:00 CLOSE OF DAY DAY 2 09:00 CONDUCT OF THE WORK AND QUALITY CONTROL Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 DEVIATION MANAGEMENT The expectations around deviations and CAPA are discussed. 10:15 WORKSHOP 3 - CONDUCT OF THE WORK AND QUALITY CONTROL Practical work conduct and quality control issues are explored. 10:45 BREAK 11:00 WORKSHOP 3 - FEEDBACK 11:30 SOURCE DATA, DATA INTEGRITY, RECORDS AND REPORTS The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 WORKSHOP 4 - DATA, RECORDS AND REPORTS Practical problems with data, records and reports are investigated. 12:45 LUNCH 13:30 WORKSHOP 4 - FEEDBACK 14:00 QUALITY AUDIT The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 RISK MANAGEMENT How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 BREAK 15:30 REGULATORY INSPECTION The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 PANEL SESSION This panel session will address any outstanding issues raised by the delegates. 16:15 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Develop

Auditing Computerised Systems
By Research Quality Association
COURSE INFORMATION Join our comprehensive three-day programme designed as an invaluable external training opportunity for auditors, audit programme managers, and individuals subject to audits. This course is tailored to foster a deep understanding and cultivate essential skills for auditing the validation of computer systems intended for GxP environments (GLP, GCP, GMP, GDP, GPvP). Commencing with an overview of regulatory prerequisites and the system life cycle, the course swiftly transitions to focus on the pragmatic aspects of auditing computer system validation. Experience a blend of presentations, interactive discussions, and immersive practical workshops throughout the duration of the course. Delegates will benefit from practical examples of how to understand the framework of applicable regulations and guidance. * Apply risk management techniques to audit planning * Plan and conduct computerised system audits * Assess system validation documentation to verify compliance * Evaluate data integrity and security issues * Prepare for regulatory inspection. THE COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO: * Discuss and develop ideas * Solve specific problems * Understand the vulnerabilities of computerised systems * Learn how to create a compliance checklist * Link system development with good business practice. IS THIS COURSE FOR YOU? * Auditors * Audit programme managers * Individuals subject to audits. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): NICHOLA STEVENS Director and Principal Consultant, Nuncius Compliance Solutions Ltd BARRY MCMANUS Consultancy Partner, Empowerment Quality Engineering Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 WELCOME AND INTRODUCTIONS 09:45 WHY WE VALIDATE AND REGULATORY TRENDS 10:30 BREAK 10:45 AUDIT OVERVIEW, HIGH LEVEL PROCESS AND SCHEDULING 11:30 SYSTEM LIFECYCLE 12:30 LUNCH 13:15 EXERCISE 1 - AUDIT SCHEDULING 14:45 EXERCISE 1 - FEEDBACK 15:15 BREAK 15:30 VALIDATION DELIVERABLES 16:30 RISK ASSESSMENTS 17:30 CLOSE OF DAY 1 DAY 2 09:00 SUPPLIER ASSESSMENT 10:30 BREAK 10:45 EXERCISE 2 - PLANNING A SUPPLIER AUDIT 12:00 EXERCISE 2 - FEEDBACK 12:30 LUNCH 13:15 EXERCISE 3 - AUDITING A COMPUTERISED SYSTEM VALIDATION PACKAGE 15:30 BREAK 15:45 EXERCISE 3 - FEEDBACK 16:30 CHANGE CONTROL 17:15 CLOSE OF DAY DAY 3 09:00 INFRASTRUCTURE QUALIFICATION 09:45 MAINTAINING A VALIDATED STATE - OPERATIONAL PROCESSES 11:00 BREAK 11:15 EXERCISE 4 - AUDITING SYSTEMS IN OPERATIONAL USE 12:45 LUNCH 13:30 EXERCISE 4 - FEEDBACK 14:15 EXERCISE 5 - AUDITING TRAIL REVIEW 15:30 BREAK 15:45 EXERCISE 5 - FEEDBACK 16:15 COURSE OBJECTIVES SUMMARY AND ANY ADDITIONAL QUESTIONS 16:45 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 19 Points DEVELOPMENT LEVEL Develop

Educators matching "Computer Systems"
Show all 33Search By Location
- Computer Systems Courses in London
- Computer Systems Courses in Birmingham
- Computer Systems Courses in Glasgow
- Computer Systems Courses in Liverpool
- Computer Systems Courses in Bristol
- Computer Systems Courses in Manchester
- Computer Systems Courses in Sheffield
- Computer Systems Courses in Leeds
- Computer Systems Courses in Edinburgh
- Computer Systems Courses in Leicester
- Computer Systems Courses in Coventry
- Computer Systems Courses in Bradford
- Computer Systems Courses in Cardiff
- Computer Systems Courses in Belfast
- Computer Systems Courses in Nottingham







