Booking options
£74
£74
On-Demand course
1 hour 30 minutes
Intermediate level
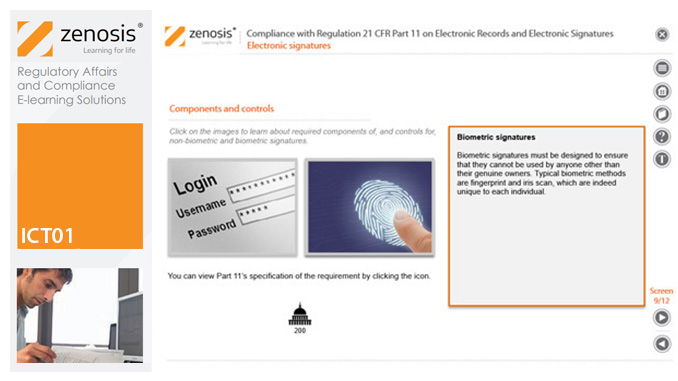
Part 11 of Title 21 of the US Code of Federal Regulations (21CFR11) sets out requirements that computer systems must meet to satisfy the Food and Drug Administration (FDA) that electronic records and electronic signatures provided by those systems are trustworthy and reliable to the same extent as paper counterparts. The regulation sets out controls and procedures which need to be established and followed for relevant computer systems in FDA-regulated environments. An FDA-regulated environment is a ‘GxP’ environment operated by an organisation involved in activities leading to the marketing of drugs or medical devices in the USA; examples are drug manufacturing sites, medical device manufacturing sites, analytical laboratories, clinical investigational sites, and nonclinical study laboratories.
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.
Companies that market or intend to apply for approval to market drugs or medical devices in the USA must comply with 21CFR11, whether or not they are based in the USA. Suppliers to such companies of materials, equipment, or data that are subject to FDA regulation must also comply.
