- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
HR Management and Recruitment - Endorsed Certificate
By Imperial Academy
Level 5- Two Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

HACCP and Food Allergen Awareness
By Imperial Academy
Level 3 & 5 Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

HR (Change Management and Recruitment Consultant)
By Imperial Academy
Level 5-Two Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

Construction Management & Cost Estimation - QLS Endorsed Diploma
By Imperial Academy
Level 5 & 7 Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

QLS Endorsed Digital Marketing and Social Media Marketing
By Imperial Academy
Level 3 & 7 Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

Information Management and GDPR
By Imperial Academy
Level 3 - Two Endorsed Training | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

Agricultural Science Level 3 & 5
By Imperial Academy
Level 5 QLS Endorsed Course | Endorsed Certificate Included | Plus 5 Career Guided Courses | CPD Accredited

Our One-Day Sleep Awareness Workshop (6 months to 6 years)
By Millpond Childrens Sleep Clinic

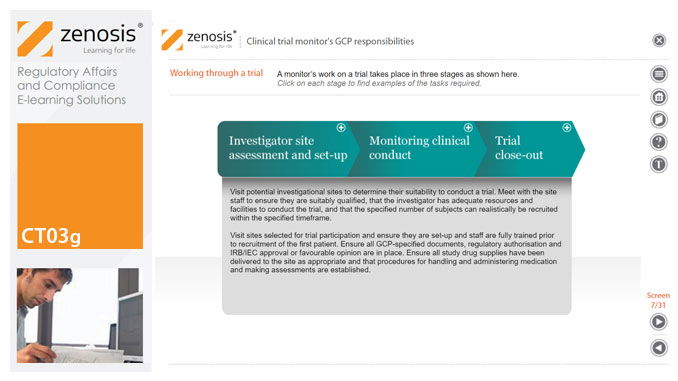
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
NEBOSH IIRSM Certificate in Managing Risk ~ Via eLearning
5.0(18)By Woodward Safety Health And Environment Ltd
This qualification provides a comprehensive overview of risk and risk management. It is a great way for health and safety professionals to expand their knowledge of risk beyond health and safety.
