- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
799 Manufacturing courses delivered Online
Level 4 Diploma in Accounting and Business (Fast Track mode)
4.0(2)By London School Of Business And Research

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Advanced Diploma in CNC (Computer Numerical Control) Programming for Machining
4.8(12)By Academy for Health and Fitness

GLP02 - Good Quality Control Laboratory Practice
By Zenosis
The medicinal products industry is heavily regulated by governments. Within the industry’s Good Manufacturing Practice (GMP) framework, analytical laboratories engaged in quality control (QC) of starting materials, intermediates, bulk products, finished products, and packaging need to comply with relevant GMP standards. We refer to these as Good Quality Control Laboratory Practice, or GQCLP. Regulatory authorities inspect laboratories to confirm that they meet the standards. This course explains how to comply with GQCLP, and it provides advice on laboratory work in general.

SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
Enhance your career with our Assembly Operative Skills Development Course, covering key aspects like assembly operations, tools, safety, and advanced techniques. Master the art of efficient assembly line setup, navigate manufacturing laws, and stay ahead with the latest technologies. Elevate your skills for success in today's dynamic manufacturing landscape. Enroll now for a comprehensive learning experience!

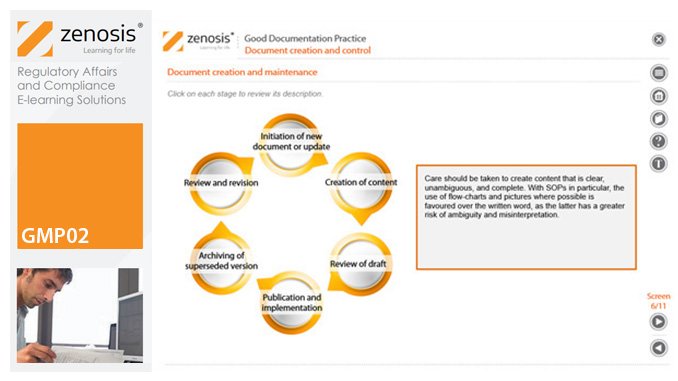
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.