Booking options
Price:
BuyContact Us£39
£39
On-Demand course
1 hour
Intermediate level
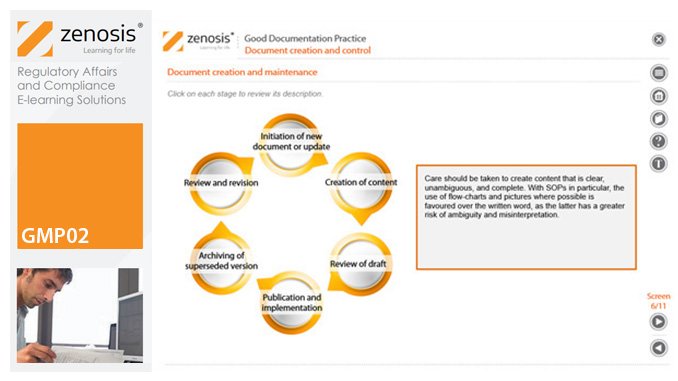
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
In this module, we emphasise the crucial importance of GDocP and we identify five principles that underpin it. We explain the functions of the various types of documents that are used and discuss how they should be created and controlled. Finally, we set out requirements for record keeping – how data are to be entered into records, corrected if necessary, and how records must be retained.
