114 Clinical Practitioner courses
Get a 10% discount on your first order when you use this promo code at checkout: MAY24BAN3X
Extended Clinical Skills for Support Workers
By Guardian Angels Training
Enhance your clinical expertise with our "Extended Clinical Skills Training" course. Designed for healthcare professionals, this course provides practical skills and knowledge for extended clinical procedures and interventions.

CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

This is a post-graduate course composed of a TCM and Acupuncture Theory Foundation Module (9 lecture days) and a Special Orthopaedic Acupuncture Module (8 lecture days) with additional 4 clinical practice days and a re- validation day. The TCM and Acupuncture Theory foundation Module leads to a Foundation Diploma in TCM and Acupuncture Level-1. The Orthopaedic Acupuncture module is designed in such a way that it can either be completed as a whole, or each of the 8 lectures can be taken as a CPD unit for therapist with special interests in the specific areas of the body. The course is specifically designed as an advanced, intensive course for acupuncturists, physiotherapists, osteopaths, chiropractors and other physical therapists who wish to broaden their knowledge and further their skills. The emphasis of the course falls on the practical application of acupuncture as a treatment for a variety of MSK conditions.

The Auditing Course
By Research Quality Association
COURSE INFORMATION Designed to develop personal proficiency in audit planning, execution and reporting, this course is meticulously crafted to refine essential audit skill sets. Through immersive scenarios focused on on-site audit conduct (with an alternative Remote Auditing Course available), participants will engage deeply in the audit process. EXTENDING EXPERTISE: Applicable across all audit types, this course builds upon and enriches the foundational concepts taught in RQA's suite of research quality assurance courses. From 'Research Quality Assurance for Good Laboratory Practice' to 'Good Clinical Practice Auditing – Principles and Practice' and 'Good Manufacturing Practice for Investigational Medicinal Products,' this programme extends the scope of learning. RELEVANCE AND VALUE: Relevant to any area of regulated research and development, this course shines particularly in contexts mandating a quality system for audit. Participants with prior audit experience will gain maximum value from this course. KEY BENEFITS: ENRICH YOUR SKILL SET TO: * Navigate audit processes encompassing planning, execution, reporting, and follow-up * Embrace a personalised approach fostering positive audit outcomes * Analyse evidence and present cohesive audit findings * Recognise the pivotal role of audits in driving continual improvement. INTERACTIVE LEARNING: Structured to foster dynamic engagement, this course encourages delegates to: * Engage in discussions, idea development, and problem-solving * Exchange invaluable information and experiences. HANDS-ON EXPERIENCE: A highlight of this course is the series of practical workshops, where delegates work in small syndicate groups, applying the acquired skills from lectures into real-world scenarios. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): ANDREW WADDELL Founder Director, Tower Mains Ltd ROSEMARY ICHABA Senior QA Associate, Tower Mains Ltd CATE OVINGTON Director, The Knowlogy Group Ltd JEAN MCWILLIAM Associate Director, Alexion View pop up [https://www.therqa.com/learn-develop-connect/courses-and-events/events/course/the-auditing-course/#] -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:45 REGISTRATION 09:00 WELCOME AND COURSE OBJECTIVES 09:10 WHAT IS 'AUDIT'? Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 09:30 AUDITS AND THEIR PURPOSE The concepts of quality assurance, quality control, quality management and audit are discussed. 10:30 BREAK 10:45 AUDIT PLANNING The requirements for an effective audit programme and individual audit plans. 11:30 WORKSHOP 1 - GETTING THE AUDIT STARTED Planning for the audit. 12:25 WORKSHOP 1 - FEEDBACK 12:45 LUNCH 13:30 WORKSHOP 2 - GETTING THE AUDIT STARTED Arranging the opening meeting. 13:50 WORKSHOP 2 - FEEDBACK Audit initiation. Review and discussion of the role of the opening meeting. 14:25 AUDITING TECHNIQUES (1) - DATA AND DOCUMENTATION Techniques for the conduct of data and report audits are investigated. 14:55 BREAK 15:10 WORKSHOP 3 - DATA AND DOCUMENTATION AUDIT Conducting an audit of a data package and supporting documentation. 17:15 CLOSE OF DAY DAY 2 09:00 AUDITING TECHNIQUES (2) - THE PEOPLE Questioning techniques which get the required information from the auditee. 09:45 LIVE AUDIT ROLE PLAY Auditor and auditee behaviours are explored and strategies developed for successful audit interactions. 10:15 BREAK 10:35 AUDIT CLOSING MEETING An exploration of audit closing meetings. 11:00 WORKSHOP 4 - AUDIT OBSERVATIONS AND PREPARING FOR THE CLOSING MEETING Reviewing and categorising your observations and getting ready to present your case. 11:45 WORKSHOP 4 - FEEDBACK 12:30 AUDIT REPORTS The content and distribution of an effective audit report are investigated and the importance of effective written communication is discussed. 13:00 LUNCH 13:45 WORKSHOP 5 - AUDIT REPORTS AND FOLLOW-UP Mechanisms for promoting effective corrective and preventive action. Critical review of an audit report example. 14:30 WORKSHOP 5 - FEEDBACK 14:55 CORRECTIVE AND PREVENTIVE ACTION AND FOLLOW-UP The auditor's role in monitoring responses to audit and the corrective and preventive actions promised is explored. 15:20 PANEL SESSION An opportunity to get answers to outstanding questions. 15:30 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. CPD POINTS 14 Points DEVELOPMENT LEVEL Develop

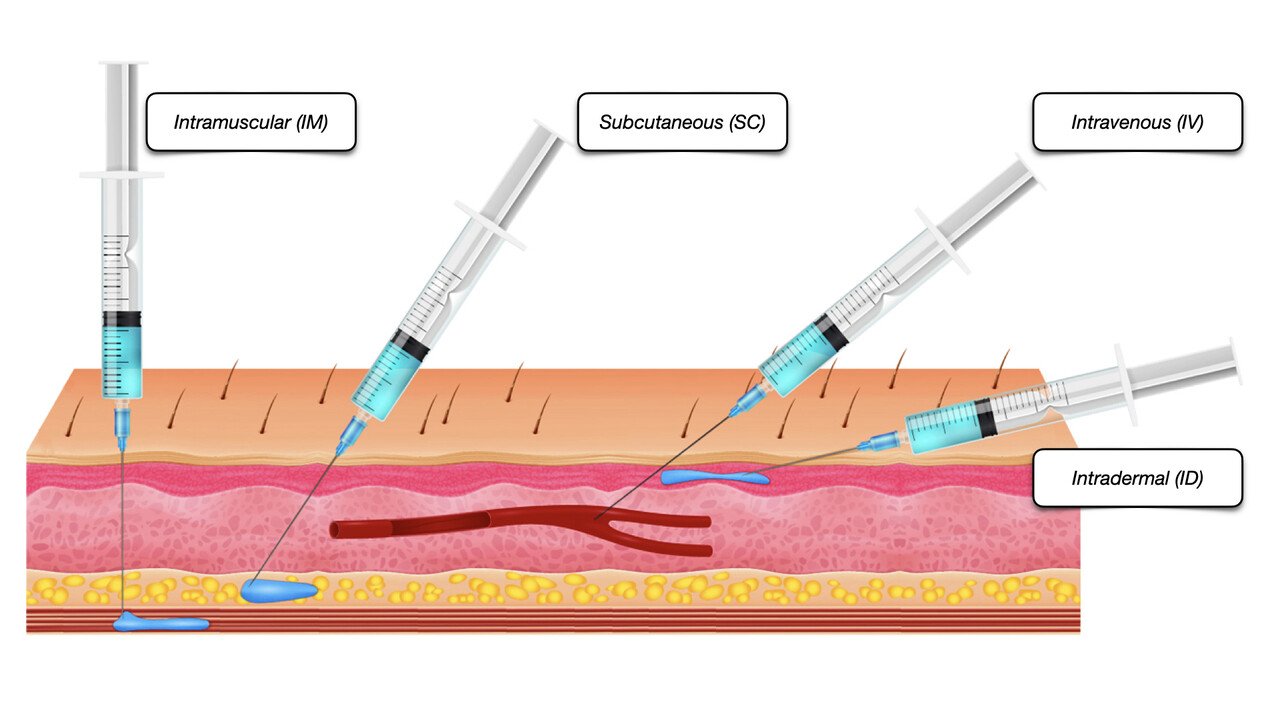
Peripheral Intravenous Cannulation (Peripheral vascular access device)
By Guardian Angels Training
Gain the knowledge and skills to safely insert and manage peripheral IV cannulas with our training course for healthcare professionals. Ideal for nurses, medical personnel, and other practitioners.
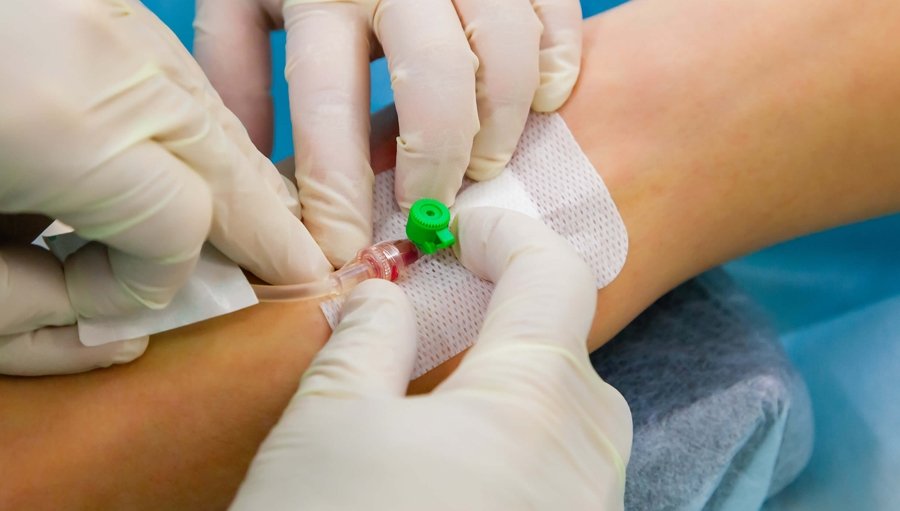
An Understanding of Injectable LHRH agonists
By Guardian Angels Training
Gain comprehensive knowledge on injectable LHRH agonists with our course. Learn about mechanisms, clinical applications, administration techniques, and more.
RCN Accredited Asthma and COPD Course NORFOLK ICB ONLY
By BBO Training
'RCN Accredited Asthma and COPD' Course This course is a collaborative offering between your employer and BBO Training Ltd., designed to equip experienced healthcare professionals working in primary care settings with a comprehensive and holistic approach to managing patients with asthma and COPD. Even for those already holding a Diploma in either of these areas, the course has proven to be highly valuable, as indicated by positive evaluations from healthcare professionals. Course Aim The primary objective of this course is to provide qualified healthcare professionals with the necessary knowledge and skills to deliver effective asthma and COPD services in collaboration with their medical colleagues within the community healthcare setting. The course aims to raise awareness of these chronic conditions within the community, ensuring safe practice through the utilization of Patient Group Directions (PGDs) and protocols where appropriate. Participants will be clinically and theoretically prepared to establish and manage review and diagnostic clinics within their competency framework. Furthermore, the course emphasizes the clear differentiation between asthma and COPD. Clinical Practice Program and Competencies Throughout the course, you, along with your primary mentor, will organise and validate your clinical engagements, both under supervision and independently. By the time of your final assessment, these forms should be fully completed and ready for submission, accompanied by both your and your mentor's evaluations. Additionally, maintaining a log of all clinical hours and interactions is required, with a minimum of 36 hours. Teaching and Learning Approach Upon completing this course, students will have the opportunity to: 1. Participate in various sessions, including workshops, simulations, discussions, seminars, and tutorials, which can be conducted either virtually or in-person. 2. Gain practical experience in asthma and COPD management within their local healthcare settings. 3. Develop an in-depth understanding of treatments, including their composition, efficacy, indications, contraindications, and mechanisms of action, through clinical practice. 4. Enhance advisory skills related to respiratory care on a broader scale. 5. Attend dedicated sessions for clinical practice. 6. Document a minimum of 36 hours of supervised clinical practice and maintain an ongoing reflective diary. Expected Course Content Workshops, Discussion Groups, Lectures, and Assessment: 40 hours Clinical Practice: A minimum of 36 hours Visits and Private Study: 44 hours Private study time is essential for reading, conducting factual research on treatments through online sources, and accessing the library for literature searches. This time will facilitate writing, referencing, completing your reflective diary, and preparing for the final assessment. Assessment Successful completion of all components is required. Components 2 to 4 must be passed to complete the course. A resit option is available for any component that is not initially passed, limited to one attempt. 1. Reflective Diary: This ongoing document, produced by the student throughout the course, is validated by both the student and mentor. While not directly marked, its completion is integral to your portfolio of evidence. 2. Clinical Outcomes: These must be validated and passed by your mentor, with all outcomes achieved, along with a log of clinical hours. 3. VIVA: A minimum pass mark of 50% is required. 4. Test of Knowledge: A minimum pass mark of 50% is necessary (conducted at the end of the initial study days). Learning Outcomes Upon completion of this course, students will be capable of: 1. Evaluating and showcasing clinical competence through a reflective diary/log. 2. Demonstrating clinical assessment and treatment proficiency in a VIVA examination and knowledge assessment. 3. Effectively educating individuals about self-management and enhancing their understanding of their condition. 4. Displaying sensitivity and competence in obtaining comprehensive patient histories. 5. Adapting care for diverse patient groups and their unique needs. 6. Fulfilling professional responsibilities, including meticulous record-keeping and maintaining confidentiality. 7. Teaching the use of various inhalers and assessment equipment. 8. Developing fundamental assessment and examination techniques for respiratory conditions. 9. Operating with Patient Group Directions and protocols for asthma and COPD care. 10. Assessing, planning, implementing, and evaluating individual patient needs comprehensively.
GCP Good Clinical Practice
By NBScience
GCP Good Clinical Practice

End of Life Care Training, Syringe Driver Management Training, Verification of Death Training
5.0(22)By Wren Healthcare Ltd
These topics are available as individual sessions or we can deliver all three as a full day’s training for Registered Practitioners. Facilitated by registered clinicians with extensive experience.

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

Search By Location
- Clinical Practitioner Courses in London
- Clinical Practitioner Courses in Birmingham
- Clinical Practitioner Courses in Glasgow
- Clinical Practitioner Courses in Liverpool
- Clinical Practitioner Courses in Bristol
- Clinical Practitioner Courses in Manchester
- Clinical Practitioner Courses in Sheffield
- Clinical Practitioner Courses in Leeds
- Clinical Practitioner Courses in Edinburgh
- Clinical Practitioner Courses in Leicester
- Clinical Practitioner Courses in Coventry
- Clinical Practitioner Courses in Bradford
- Clinical Practitioner Courses in Cardiff
- Clinical Practitioner Courses in Belfast
- Clinical Practitioner Courses in Nottingham