4 Clinical Practitioner courses in Nottingham
Get a 10% discount on your first order when you use this promo code at checkout: MAY24BAN3X
Extended Clinical Skills for Support Workers
By Guardian Angels Training
Enhance your clinical expertise with our "Extended Clinical Skills Training" course. Designed for healthcare professionals, this course provides practical skills and knowledge for extended clinical procedures and interventions.

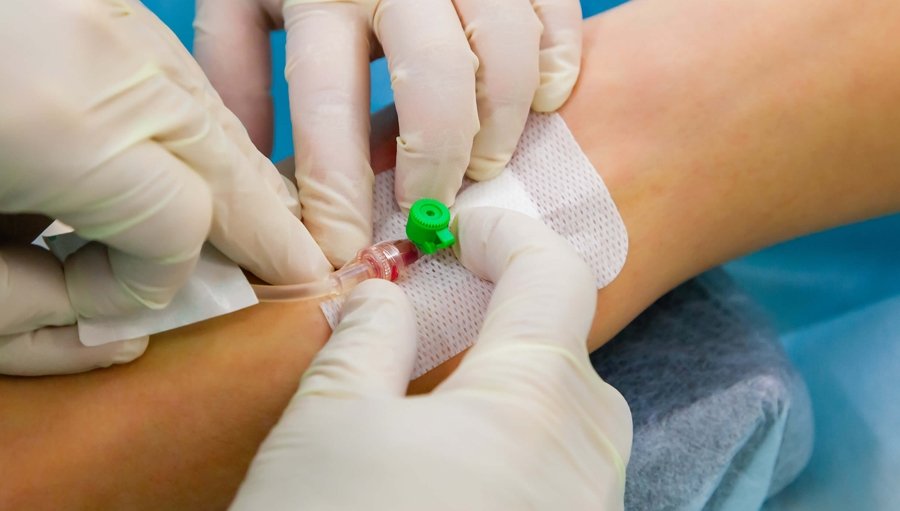
Peripheral Intravenous Cannulation (Peripheral vascular access device)
By Guardian Angels Training
Gain the knowledge and skills to safely insert and manage peripheral IV cannulas with our training course for healthcare professionals. Ideal for nurses, medical personnel, and other practitioners.
An Understanding of Injectable LHRH agonists
By Guardian Angels Training
Gain comprehensive knowledge on injectable LHRH agonists with our course. Learn about mechanisms, clinical applications, administration techniques, and more.
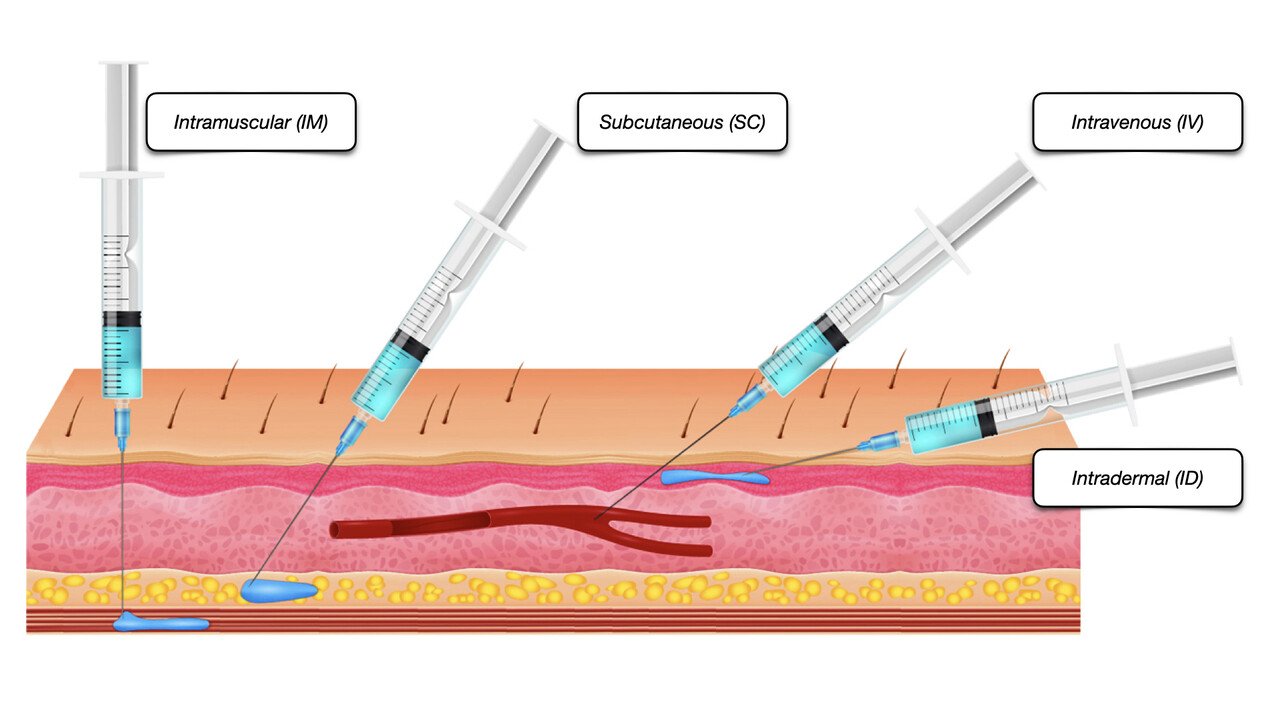
Informed Consent in Clinical Practice
By M&K Update Ltd
This one-day course equips you to fully understand your role in the consenting process and matters that you need to take into consideration in order for the consent to be valid.

Online Options
Show all 101The Remote Auditing Course
By Research Quality Association
COURSE INFORMATION Join us for a two-day immersive course crafted to equip participants with an in-depth understanding of remote audit methodologies. Delve into the nuances between remote and face-to-face audits, dissecting their respective strengths and limitations. Through practical scenarios tailored for remote audit conduct, this course stands as an essential counterpart to our on-site audits course The Auditing Course. WHO SHOULD ATTEND: Applicable across various domains of regulated research and development, this course proves invaluable in contexts requiring a quality system for audit. Participants with firsthand audit experience stand to gain the most benefit from this programme. EXPANDING ON PREVIOUS LEARNING: This course extends its relevance to all forms of audits and further amplifies concepts explored in RQA's suite of research quality assurance courses, including: 'Research Quality Assurance for Good Laboratory Practice,' 'Good Clinical Practice Auditing – Principles and Practice,' and 'Good Manufacturing Practice for Investigational Medicinal Products.' BENEFITS INCLUDE IMPROVED: * Understand the processes of planning, conducting, reporting and follow-up of audits * Recognising the importance of personal approach in developing positive audit outcomes * Ability to analyse evidence and present logical audit findings * Appreciate the importance of audit in continuing improvement. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO: * Discuss and develop ideas * Solve problems * Exchange information. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): ANDREW WADDELL Founder Director, Tower Mains Ltd ROSEMARY ICHABA Senior QA Associate, Tower Mains Ltd CATE OVINGTON Director, The Knowlogy Group Ltd JEAN MCWILLIAM Associate Director, Alexion -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 COURSE REGISTRATION 09:15 WELCOME AND INTRODUCTIONS 09:35 INTRODUCTION TO AUDITS Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 10:05 INTRODUCTION TO REMOTE AUDITS Presentation to introduce the major differences between face-to-face audits and remote audits. The major elements of audits will be examined to determine where there may be differences. 10:30 BREAK 10:45 WORKSHOP 1 - REMOTE AUDITS This workshop will examine participants experience or understanding of the differences between face-to-face and remote audits. 11:15 WORKSHOP 1 - FEEDBACK 11:35 AUDIT PREPARATION The essential steps in preparation for audits will be discussed with emphasis on remote audits. 12:05 WORKSHOP 2 - REMOTE AUDIT PREPARATION Factors relating to the preparation for remote audits will be considered in discussion groups. 12:35 WORKSHOP 2 - FEEDBACK 12:50 LUNCH 13:30 AUDIT LOGISTICS Preparation for the audit includes many arrangements other than the audit content. 14:00 WORKSHOP 3 - AUDIT LOGISTICS Delegates will discuss some of the issues that may occur during the set up and conduct of remote audits and how to resolve them. 14:30 WORKSHOP 3 - FEEDBACK 14:45 BREAK 15:00 THE OPENING MEETING Presentation regarding the importance and content of the opening meeting. 15:20 WORKSHOP 4 - OPENING MEETING Delegates will consider the practicalities of arranging and conducting an opening meeting. 15:45 WORKSHOP 4 - FEEDBACK 16:10 AUDIT CONDUCT TOOLS Practical advice on preparation of some of the tools that will be valuable in the conduct of audits. 16:40 QUESTIONS AND ANSWERS An overview of the first day and a chance to ask questions 17:00 CLOSE OF DAY DAY 2 09:00 REFLECTIONS ON DAY 1 Recap on the topics covered and a chance for delegates to raise points. 09:15 GATHERING EVIDENCE Presentation on challenges of gathering evidence and conducting interviews during remote audits. 09:45 WORKSHOP 5 - REMOTE INTERVIEWS Scenarios of different remote interviews will be played and delegates assess the strengths and weaknesses of each approach. 10:15 BREAK 10:30 CREATING AUDIT FINDINGS Ideas regarding how to create good audit findings will be discussed during this short presentation. 10:50 WORKSHOP 6 - CREATING AUDIT FINDINGS Delegates will be provided with examples of evidence from an audit. They will discuss these and create their audit findings for presentation at the closing meeting. 11:35 THE CLOSING MEETING The content and conduct of the closing meeting will be discussed. 11:55 WORKSHOP 7(A) - PREPARING FOR THE CLOSING MEETING The findings from workshop 6 will be reviewed and a Lead Auditor selected to provide feedback in workshop 7(b) 12:15 WORKSHOP 7(B) - THE CLOSING MEETING The Lead Auditor from each group will hold a meeting to present the results (role play). 12:45 LUNCH 13:30 THE AUDIT REPORT This presentation will cover not only the essential contents of the audit report but also how to write the report in a manner that will generate the most positive reaction. 14:00 WORKSHOP 8 - THE AUDIT REPORT Delegates will be provided with an audit report from an audit conducted remotely and will conduct a peer review of the report in their breakout groups. 15:00 WORKSHOP 8 - FEEDBACK 15:20 BREAK 15:35 AUDIT CLOSURE Presentation on how to conclude the audit with reference to post-audit activities. 15:55 OPEN FORUM 16:30 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam.

Peer Supervision for Clinical Hypnotherapists: Peer supervision for clinical hypnotherapists fosters professional growth through enhanced reflective practice, continuous learning, emotional support, feedback and validation, ethical guidance, networking, and professional accountability. - **Enhanced Reflective Practice**: Facilitates self-reflection, helping hypnotherapists identify strengths, weaknesses, and areas for improvement through peer discussions. - **Continuous Learning**: Expands knowledge by sharing innovative techniques, research findings, and emerging trends, promoting ongoing professional development. - **Emotional Support**: Provides a supportive space for therapists to share experiences and receive emotional support, addressing the emotional demands of the profession. - **Feedback and Validation**: Offers constructive criticism and fresh perspectives, aiding in skill refinement and improved clinical practice. - **Ethical Guidance**: Allows discussion of ethical dilemmas and collaborative solutions, ensuring adherence to professional standards. - **Networking and Collaboration**: Builds professional networks, leading to collaboration, referrals, and partnerships. - **Professional Accountability**: Encourages high standards and self-reflection through peer discussions, enhancing practice quality.

Medical Terminology Training Courses - Level 2
By Mediterm Training
This course leads to the Mediterm Intermediate Award in Medical Terminology (Level 2), studied over approximately 12 weeks (taking more or less time dependent on learner requirements). This course is suitable for those already working in healthcare or those who wish to start a new career in healthcare.
COPD Update
By BBO Training
COURSE OVERVIEW: COPD UPDATE DAY FOR PRIMARY CARE PRACTITIONERS JOIN US FOR A COMPREHENSIVE AND ENRICHING COPD UPDATE DAY, SPECIFICALLY DESIGNED FOR REGISTERED HEALTHCARE PROFESSIONALS WHO ARE CURRENTLY PRACTICING IN THE FIELD OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD). THIS COURSE AIMS TO ENHANCE YOUR EXISTING KNOWLEDGE AND SKILLS, KEEPING YOU UP-TO-DATE WITH THE LATEST DEVELOPMENTS IN COPD MANAGEMENT. DURING THIS INFORMATIVE DAY, YOU WILL DELVE INTO THE ESSENTIALS OF COPD, STARTING FROM ITS FUNDAMENTAL DEFINITION AND DIAGNOSTIC CRITERIA TO THE LATEST INVESTIGATIVE APPROACHES. THE COURSE WILL COVER BOTH NON-PHARMACOLOGICAL AND PHARMACOLOGICAL MANAGEMENT STRATEGIES, WITH A SPECIAL EMPHASIS ON PRACTICAL INHALER TECHNIQUES. WE WILL ALSO EXPLORE THE CURRENT GOLD & NICE GUIDELINES, DISCUSSING THEIR IMPLICATIONS AND PRACTICAL APPLICATIONS IN CLINICAL PRACTICE. A SIGNIFICANT FOCUS WILL BE ON THE MANAGEMENT OF EXACERBATIONS AND LUNG ATTACKS, INCLUDING STRATEGIES FOR PREVENTION AND EFFECTIVE MANAGEMENT TECHNIQUES, WITH A PARTICULAR EMPHASIS ON PATIENT SELF-MANAGEMENT. UNDERSTANDING THE COMPLEXITIES AND CARE REQUIREMENTS OF END-STAGE COPD WILL ALSO BE A KEY ASPECT OF THIS COURSE. THE DAY WILL CONCLUDE WITH INTERACTIVE CASE STUDIES, ALLOWING YOU TO APPLY YOUR REFRESHED KNOWLEDGE IN REAL-WORLD SCENARIOS, ENCOURAGING THE SHARING OF EXPERIENCES AND BEST PRACTICES AMONG YOUR PEERS. THIS COURSE OFFERS A DYNAMIC AND SUPPORTIVE ENVIRONMENT FOR PROFESSIONALS SEEKING TO REFINE THEIR APPROACH TO COPD CARE, COMBINING THEORETICAL LEARNING WITH PRACTICAL APPLICATION LEARNING OUTCOMES: DEFINING AND DIAGNOSING COPD REVIEW AND UPDATE THE DEFINITION AND UNDERSTANDING OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD). REFRESH KNOWLEDGE ON DIAGNOSTIC CRITERIA AND INVESTIGATIONS FOR COPD. NON-PHARMACOLOGICAL MANAGEMENT EXPLORE VARIOUS NON-PHARMACOLOGICAL APPROACHES TO MANAGING COPD. GUIDELINES AND BEST PRACTICES DELVE INTO THE GOLD & NICE GUIDELINES AND THEIR PRACTICAL APPLICATIONS IN COPD MANAGEMENT. PHARMACOLOGY AND INHALER TECHNIQUES UPDATE KNOWLEDGE ON PHARMACOLOGICAL TREATMENTS FOR COPD. MASTER INHALER TECHNIQUES AND UNDERSTAND THEIR CRITICAL ROLE IN COPD MANAGEMENT. LUNG ATTACKS AND EXACERBATION MANAGEMENT LEARN STRATEGIES TO REDUCE THE RISK OF LUNG ATTACKS/EXACERBATIONS. DISCUSS MANAGEMENT TECHNIQUES FOR EXACERBATIONS, INCLUDING PATIENT SELF-MANAGEMENT. MANAGING END-STAGE DISEASE UNDERSTAND THE COMPLEXITIES OF MANAGING END-STAGE COPD. FOCUS ON PALLIATIVE CARE AND IMPROVING QUALITY OF LIFE FOR PATIENTS WITH ADVANCED COPD. CASE STUDIES AND PRACTICAL APPLICATION APPLY KNOWLEDGE THROUGH INTERACTIVE CASE STUDIES. ENCOURAGE SHARING OF EXPERIENCES AND BEST PRACTICES AMONG PARTICIPANTS. This COPD Update Day ensures that seasoned primary care practitioners remain up-to-date with the latest advancements in COPD management, fostering an environment of continuous learning, collaboration, and patient-centered care.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
COURSE INFORMATION Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. HOW IS THIS COURSE RUN? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. WHAT WILL I LEARN? * A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments * Solid grounding in quality assurance activities aligned with regulatory standards * Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues * Clarity on the roles and responsibilities inherent to clinical trials auditing * Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents * A nuanced understanding of regulatory inspectors' activities * Expanded professional networks to propel your auditing career forward. BENEFITS INCLUDE: * A clear understanding of the role of the auditor under Good Clinical Practice improved audits * Improved Good Clinical Practice compliance for your clinical trials. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO: * Discuss and develop ideas * Solve specific problems * Examine particular aspects of Good Clinical Practice. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): ROSEMARIE CORRIGAN EVP Global Quality, Worldwide Clinical Trials CATHY DOVE Director and Owner, Dove Quality Solutions JULIE KELLY Associate Director, Clinical Quality Assurance, Corcept Therapeutics SUSANA TAVARES Director of Research Quality Assurance, - -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 12:30 REGISTRATION 13:00 WELCOME AND OBJECTIVES FOR THE FIRST DAY OF THE COURSE 13:30 LAYING THE FOUNDATIONS Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 BREAK 15:00 PATIENT PROTECTION Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 WORKSHOP 1 - CASE STUDY ON INFORMED CONSENT 16:45 END OF DAY QUESTIONS AND ANSWERS 17:00 CLOSE OF DAY DAY 2 08:50 QUESTIONS AND ANSWERS FROM DAY 1 09:00 EFFECTIVE SITE AUDITS The procedures involved in selecting and setting up audits at investigator sites. 09:40 WORKSHOP 1 - PLANNING THE EFFECTIVE AUDIT 10:30 BREAK 10:45 SOURCE DATA VERIFICATION The need for and purpose of verifying data. 11:25 WORKSHOP 2 - SOURCE DATA VERIFICATION 12:30 LUNCH 13:30 IMP MANAGEMENT The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 CRITICAL DOCUMENT AUDITS The conduct of other study specific audits including protocols, databases and reports. 15:00 BREAK 15:15 NON-COMPLIANCE Determining the acceptability of data. 16:00 FRAUD - FACT OR FICTION? How to identify fraud and its consequences 16:45 END OF DAY QUESTIONS AND ANSWERS 17:00 CLOSE OF DAY DAY 3 08:50 QUESTIONS AND ANSWERS FROM DAYS 1 AND 2 09:00 AUDITING THIRD PARTIES A review of audits of contract research organisations. 10:00 SYSTEM AUDITS The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 BREAK 11:00 WORKSHOP 3 - PROCESS MAPPING 11:45 EFFECTIVE AUDITS Where theory meets reality. 12:30 LUNCH 13:20 AUDIT REPORTS - CLOSING THE LOOP An examination of the processes which follow the evidence gathering phase of the audit. 14:20 WORKSHOP 4 - AUDIT REPORTS Audit reports, corrective and preventive action. 15:00 BREAK 15:10 REGULATORY INSPECTION Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 FINAL QUESTIONS AND ANSWERS 16:10 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 17 Points DEVELOPMENT LEVEL Develop

Implementing Good Clinical Laboratory Practice
By Research Quality Association
COURSE INFORMATION Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. IS THIS COURSE FOR YOU? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. THIS COURSE WILL GIVE YOU: * Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) * Insight into the seamless integration of GCLP within clinical programmes (GCP) * Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories * The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) * Access to a seasoned panel of speakers with extensive expertise * A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. ENGAGE IN: * Lively discussions to foster ideas * Problem-solving sessions targeting specific challenges * Detailed exploration of specific aspects within the realms of GCP and GCLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): VANESSA GRANT -, - LOUISE HANDY Director, Handy Consulting Ltd TIM STILES Consultant, Qualogy Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:50 REGISTRATION 09:00 WELCOME AND INTRODUCTION 09:20 GOOD CLINICAL PRACTICE AND THE REQUIREMENTS OF GOOD CLINICAL LABORATORY PRACTICE A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 SAFETY AND ETHICAL CONSIDERATION Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 BREAK 10:55 ORGANISATION AND PERSONNEL RESPONSIBILITIES WITHIN GCP AND THE LABORATORY The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 STAFF TRAINING AND TRAINING RECORDS Personnel records of training and competency assessments are discussed. 11:45 LABORATORY FACILITIES, EQUIPMENT AND MATERIALS Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 LUNCH 13:15 WORKSHOP 1 - FACILITIES, EQUIPMENT AND RESPONSIBILITIES Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 WORKSHOP 1 - FEEDBACK 14:15 COMPUTER SYSTEMS VALIDATION Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 TRIAL PROTOCOLS, ANALYTICAL PLANS During this session we examine the purpose, content, control and change of these important documents. 15:30 BREAK 15:45 WORKSHOP 2 - SOPS, CLINICAL PROTOCOLS, ANALYTICAL PLANS AND VALIDATION The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 WORKSHOP 2 - FEEDBACK 17:00 CLOSE OF DAY DAY 2 09:00 CONDUCT OF THE WORK AND QUALITY CONTROL Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 DEVIATION MANAGEMENT The expectations around deviations and CAPA are discussed. 10:15 WORKSHOP 3 - CONDUCT OF THE WORK AND QUALITY CONTROL Practical work conduct and quality control issues are explored. 10:45 BREAK 11:00 WORKSHOP 3 - FEEDBACK 11:30 SOURCE DATA, DATA INTEGRITY, RECORDS AND REPORTS The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 WORKSHOP 4 - DATA, RECORDS AND REPORTS Practical problems with data, records and reports are investigated. 12:45 LUNCH 13:30 WORKSHOP 4 - FEEDBACK 14:00 QUALITY AUDIT The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 RISK MANAGEMENT How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 BREAK 15:30 REGULATORY INSPECTION The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 PANEL SESSION This panel session will address any outstanding issues raised by the delegates. 16:15 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Develop

CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

RCN Accredited Asthma and COPD Course NORFOLK ICB ONLY
By BBO Training
'RCN Accredited Asthma and COPD' Course This course is a collaborative offering between your employer and BBO Training Ltd., designed to equip experienced healthcare professionals working in primary care settings with a comprehensive and holistic approach to managing patients with asthma and COPD. Even for those already holding a Diploma in either of these areas, the course has proven to be highly valuable, as indicated by positive evaluations from healthcare professionals. Course Aim The primary objective of this course is to provide qualified healthcare professionals with the necessary knowledge and skills to deliver effective asthma and COPD services in collaboration with their medical colleagues within the community healthcare setting. The course aims to raise awareness of these chronic conditions within the community, ensuring safe practice through the utilization of Patient Group Directions (PGDs) and protocols where appropriate. Participants will be clinically and theoretically prepared to establish and manage review and diagnostic clinics within their competency framework. Furthermore, the course emphasizes the clear differentiation between asthma and COPD. Clinical Practice Program and Competencies Throughout the course, you, along with your primary mentor, will organise and validate your clinical engagements, both under supervision and independently. By the time of your final assessment, these forms should be fully completed and ready for submission, accompanied by both your and your mentor's evaluations. Additionally, maintaining a log of all clinical hours and interactions is required, with a minimum of 36 hours. Teaching and Learning Approach Upon completing this course, students will have the opportunity to: 1. Participate in various sessions, including workshops, simulations, discussions, seminars, and tutorials, which can be conducted either virtually or in-person. 2. Gain practical experience in asthma and COPD management within their local healthcare settings. 3. Develop an in-depth understanding of treatments, including their composition, efficacy, indications, contraindications, and mechanisms of action, through clinical practice. 4. Enhance advisory skills related to respiratory care on a broader scale. 5. Attend dedicated sessions for clinical practice. 6. Document a minimum of 36 hours of supervised clinical practice and maintain an ongoing reflective diary. Expected Course Content Workshops, Discussion Groups, Lectures, and Assessment: 40 hours Clinical Practice: A minimum of 36 hours Visits and Private Study: 44 hours Private study time is essential for reading, conducting factual research on treatments through online sources, and accessing the library for literature searches. This time will facilitate writing, referencing, completing your reflective diary, and preparing for the final assessment. Assessment Successful completion of all components is required. Components 2 to 4 must be passed to complete the course. A resit option is available for any component that is not initially passed, limited to one attempt. 1. Reflective Diary: This ongoing document, produced by the student throughout the course, is validated by both the student and mentor. While not directly marked, its completion is integral to your portfolio of evidence. 2. Clinical Outcomes: These must be validated and passed by your mentor, with all outcomes achieved, along with a log of clinical hours. 3. VIVA: A minimum pass mark of 50% is required. 4. Test of Knowledge: A minimum pass mark of 50% is necessary (conducted at the end of the initial study days). Learning Outcomes Upon completion of this course, students will be capable of: 1. Evaluating and showcasing clinical competence through a reflective diary/log. 2. Demonstrating clinical assessment and treatment proficiency in a VIVA examination and knowledge assessment. 3. Effectively educating individuals about self-management and enhancing their understanding of their condition. 4. Displaying sensitivity and competence in obtaining comprehensive patient histories. 5. Adapting care for diverse patient groups and their unique needs. 6. Fulfilling professional responsibilities, including meticulous record-keeping and maintaining confidentiality. 7. Teaching the use of various inhalers and assessment equipment. 8. Developing fundamental assessment and examination techniques for respiratory conditions. 9. Operating with Patient Group Directions and protocols for asthma and COPD care. 10. Assessing, planning, implementing, and evaluating individual patient needs comprehensively.
GCP Good Clinical Practice
By NBScience
GCP Good Clinical Practice

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

Search By Location
- Clinical Practitioner Courses in London
- Clinical Practitioner Courses in Birmingham
- Clinical Practitioner Courses in Glasgow
- Clinical Practitioner Courses in Liverpool
- Clinical Practitioner Courses in Bristol
- Clinical Practitioner Courses in Manchester
- Clinical Practitioner Courses in Sheffield
- Clinical Practitioner Courses in Leeds
- Clinical Practitioner Courses in Edinburgh
- Clinical Practitioner Courses in Leicester
- Clinical Practitioner Courses in Coventry
- Clinical Practitioner Courses in Bradford
- Clinical Practitioner Courses in Cardiff
- Clinical Practitioner Courses in Belfast
- Clinical Practitioner Courses in Nottingham