- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
46 Courses
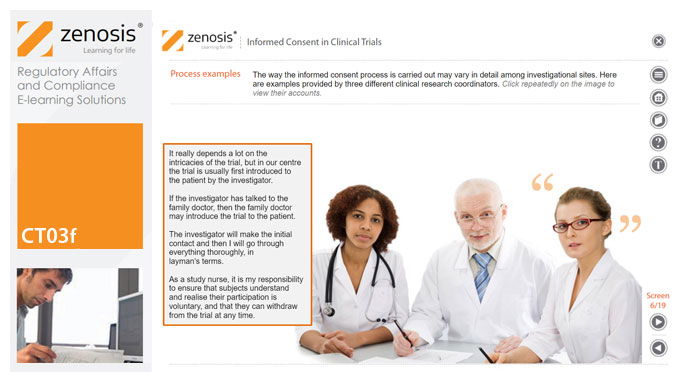
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
Genetics, Biomedical Science & Forensic Science Diploma - CPD Certified Course
By One Education

Public Health And Epidemiology Course
By Elearncollege
Level 3 Diploma in Covid19 Awareness Diploma for Healthcare Assistant - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

Clinical Data Management with SAS Programming Course
By NextGen Learning

Professional Certificate Course in UK Healthcare Regulations and Policies in London 2024
4.9(261)By Metropolitan School of Business & Management UK

Explore the intricacies of Clinical Research Administration with our comprehensive course on Protocols and Ethics. Gain expertise in trial design, ethical considerations, regulatory compliance, and more. Prepare for a future in clinical research with practical insights and knowledge. Enroll now for a rewarding journey in advancing healthcare through responsible and ethical clinical trials.

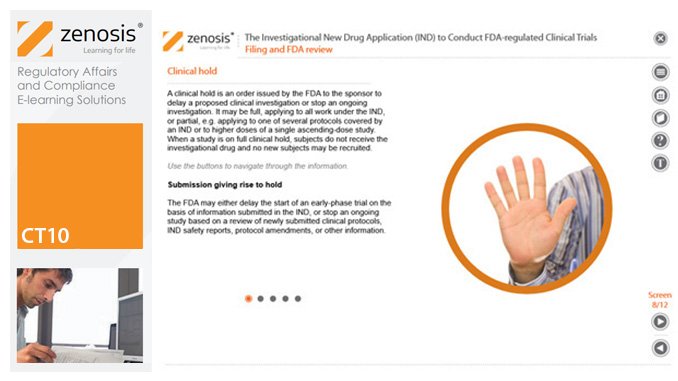
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
Search By Location
- clinical trials Courses in London
- clinical trials Courses in Birmingham
- clinical trials Courses in Glasgow
- clinical trials Courses in Liverpool
- clinical trials Courses in Bristol
- clinical trials Courses in Manchester
- clinical trials Courses in Sheffield
- clinical trials Courses in Leeds
- clinical trials Courses in Edinburgh
- clinical trials Courses in Leicester
- clinical trials Courses in Coventry
- clinical trials Courses in Bradford
- clinical trials Courses in Cardiff
- clinical trials Courses in Belfast
- clinical trials Courses in Nottingham

