- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11645 Professional Development courses delivered Online
Boundaries in the VCSE Workplace
By The Peer Partnership
This workshop is designed to support staff and organisations in the voluntary, community, and social enterprises (VCSEs) to consider, plan, and implement personal and professional boundaries in the workplace.

Brainstorm sessions
By Isobel Kent
Do you just need a quick chat to move things forward? Do you have some pressing questions and need feedback that relates directly to you and your book? Did you want to do Taking Your Book To School but are not quite ready yet? I have three half-hour brainstorm sessions available to you on Saturday 18th March at a discounted rate of £20.00 per session. Would you like one? First come first served!

Neurodiversity Workshop HR: Attraction, Recruitment, Selection
By Mpi Learning - Professional Learning And Development Provider
A four-hour workshop for HR Professionals in the area of Attraction, Selection and Recruitment of Neurodiverse people.

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

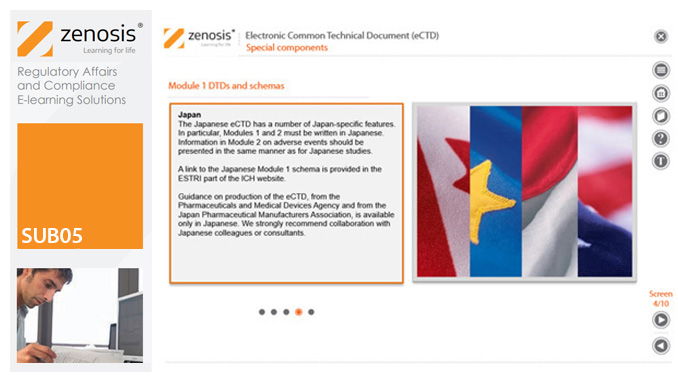
SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.
CMI Level 3 Diploma in Principles of Management and Leadership
By School of Business and Technology London

This UI (User Interface) course is for designers, front-end developers and everyone who would like to start a career in UI design. During the course you will learn how to design mobile and web applications, as well as how to work with colours, fonts, and imagery. Every week each student will get personal feedback.

ISO 13485 Quality Management Foundation
By Training Centre