- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10915 Professional Development courses delivered On Demand
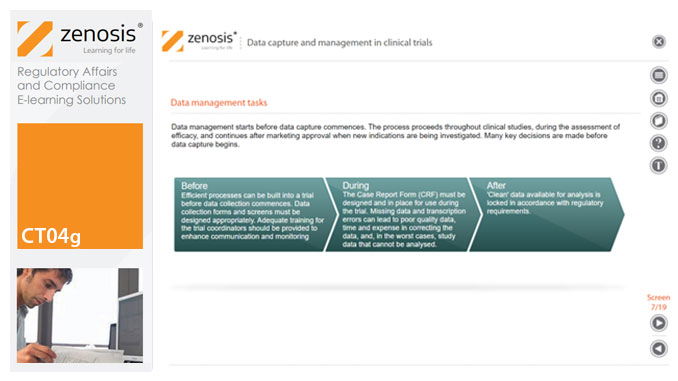
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
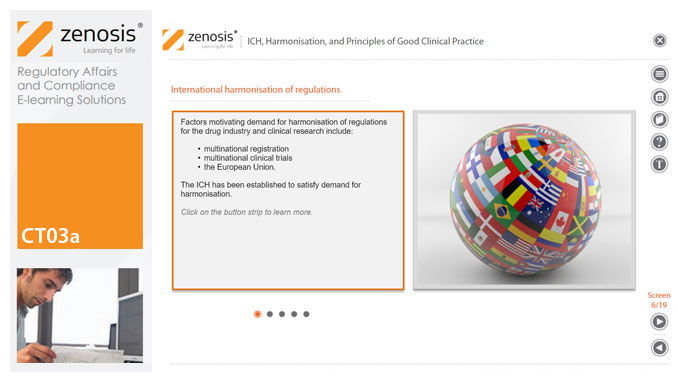
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
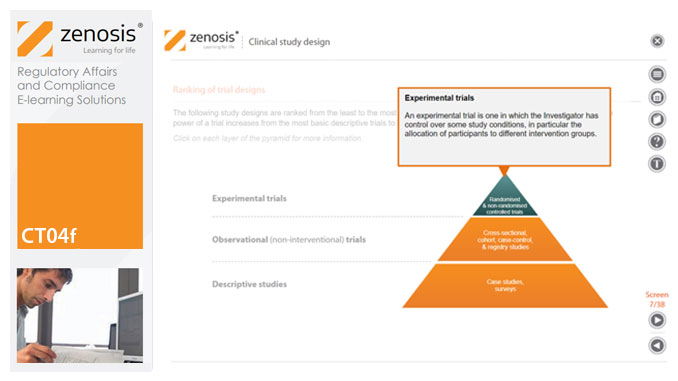
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
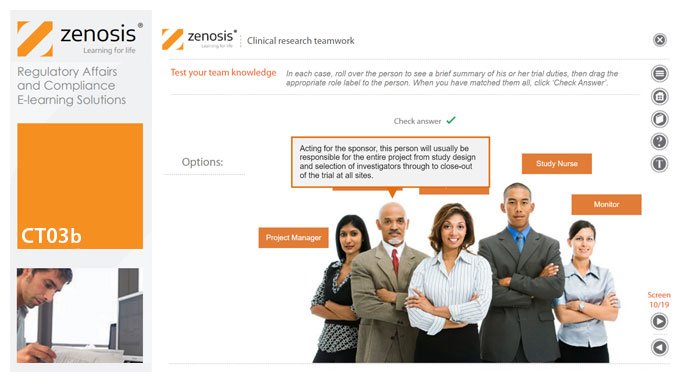
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
QUALIFI Level 7 Diploma in Law
By School of Business and Technology London

OTHM Level 7 Diploma In Health And Social Care Management
By School of Business and Technology London

QUALIFI Level 7 Diploma in Tourism and Hospitality Management
By School of Business and Technology London

Driver Safety Awareness
By Imperial Academy
Level 3 QLS Endorsed Course | CPD & CiQ Accredited | Audio Visual Training | Free PDF Certificate | Lifetime Access

Accountancy (Accountant Training)
By NextGen Learning

