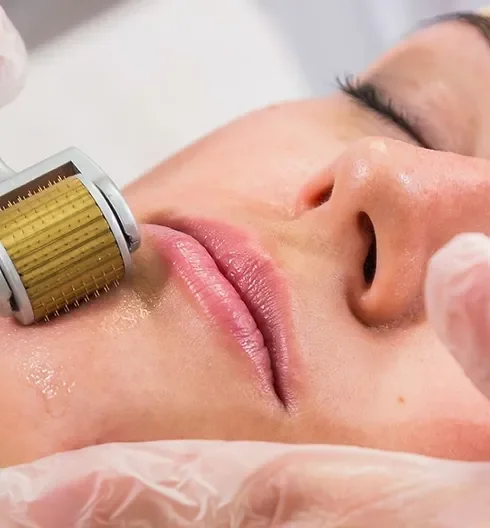
Platelet-rich Plasma (PRP) treatments Nationally Recognised Qualification No previous experience or qualifications needed Open College Network Accreditation Level 4 (as required for minimally invasive procedures) Covers standards set by HEE Employed (salon) or Self-Employed opportunities Basic understanding of English language required OPEN TO ALL APPLICANTS
THIS COURSE PACKAGE INCLUDES: 1: INTRODUCTION TO PHLEBOTOMY COURSE (GPT003) - Level 3 (Ireland Level 5) 2: ADVANCED PHLEBOTOMY COURSE (GPT005) - Level 4 (Ireland Level 6) 3: GEOPACE COMPETENCY CERTIFICATE - CPD Certified (optional with Virtual Classroom) Learn how to take blood ... train as a Phlebotomist FAST-TRACK YOUR PHLEBOTOMY TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% off - Multi-Course Discount Cover all stages from beginner through to Level 4 Available as Classroom or Virtual Classroom Complete your beginner to advanced training in 2 days Awards 2 accredited qualifications - Introduction to Phlebotomy and Advanced Phlebotomy qualifications Both courses are dually accredited (OCN & CPD) Geopace Certificate of Competency included with classroom attendance or available as an option when booking virtual classroom Covers all steps up to live blood draw Learn advanced skills and techniques Virtual Classroom options include comprehensive Practise@Home Training Kits (yours to keep) Basic understanding of English language required OPEN TO ALL APPLICANTS
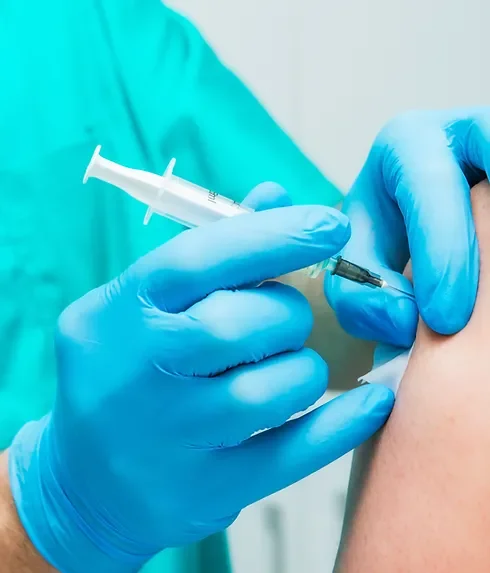
Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
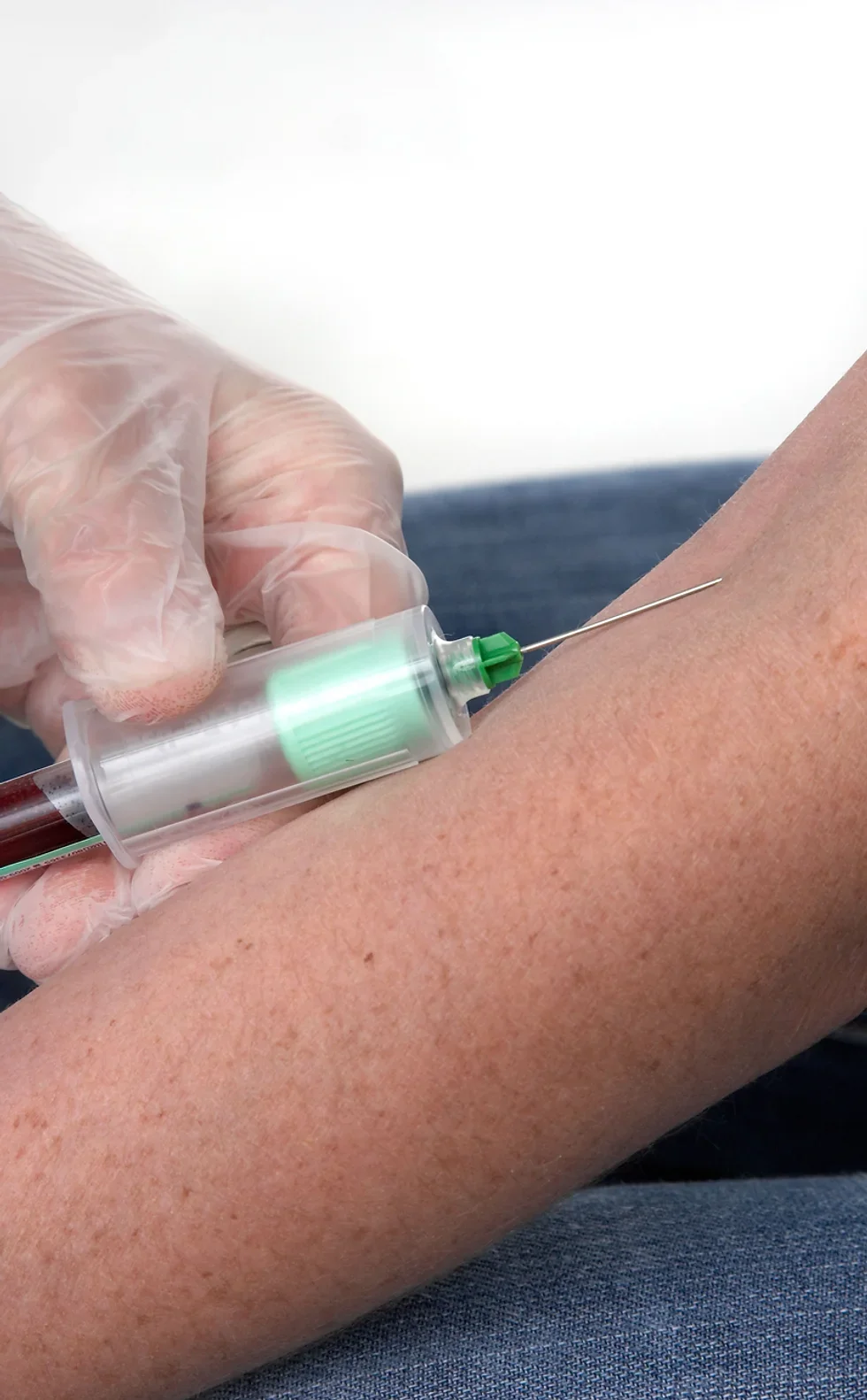
The UK's first and only Level 4 qualification in Phlebotomy (equivalent to Ireland Level 6) FDSc (Foundation Degree Level) qualification Nationally Recognised certificate Dually accredited: Open College Network and CPD Covers both aspirated and evacuated systems Covers specialised blood collection systems & methods Classroom or Virtual Classroom learning options Comprehensive Training Kit is provided when booking our Virtual Classroom option (yours to keep) Complete your training from beginner to advanced level This course either follows on from our Introduction to Phlebotomy Course or can be combined with our introductory course as part of a course package (see below) Available to candidates who have completed (or are currently enrolled to complete) our Introduction to Phlebotomy Course or have previous phlebotomy practical experience.
Quality Assurance for Good Laboratory Practice
By Research Quality Association
COURSE INFORMATION A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. WHAT WILL I LEARN? * A solid regulatory foundation underpinning quality assurance activities * Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles * Enhanced efficacy in inspections and audits * Heightened compliance with Good Laboratory Practice standards for your facility * Unique insights into governmental monitoring activities within the GLP sphere. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO * Discuss and develop ideas * Solve specific problems * Examine particular aspects of GLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): CATE OVINGTON Director, The Knowlogy Group Ltd JANE ELLISTON Senior Quality Assurance Auditor, Battelle UK SHONA ROSS Head of QA, Tower Mains Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 WELCOME AND INTRODUCTIONS 09:15 GOOD LABORATORY PRACTICE STANDARDS AND REGULATIONS An insight into the background and history of Good Laboratory Practice. 09:45 PRINCIPLES OF QUALITY ASSURANCE What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 BREAK 10:45 STANDARD OPERATING PROCEDURES GLP requirements and QA involvement. 11:30 STUDY PLANS GLP requirements and QA involvement. 12:05 QA PROGRAMME Risk based programme, what are study, process and facility audits. 13:00 LUNCH 14:00 INSPECTIONS Attitudes, techniques and attributes. 14:40 WORKSHOP 1 - FACILITY AND PROCESS INSPECTIONS An exercise in inspection planning and preparation for inspections. 15:15 BREAK 15:30 WORKSHOP 1 - FEEDBACK 15:45 THE AUDITOR AND AUDIT CONDUCT Attitudes, attributes and techniques. 16:30 PANEL SESSION An opportunity for delegates to put questions to the panel of speakers. 17:15 CLOSE OF DAY DAY 2 09:00 WORKSHOP 2 - A MOCK AUDIT 10:45 BREAK 11:00 WORKSHOP 2 - FEEDBACK 11:30 AUDITING THE STUDY REPORT Techniques and methods for the QA audit of the study report. 12:00 RECORD KEEPING AND DATA The impact of GLP on data and records management. 12:40 LUNCH 13:25 DATA INTEGRITY A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 WORKSHOP 3 - AMENDMENTS TO STUDY PLAN AND DEVIATIONS FROM THE PLAN What are they? What is the difference between them? How are they controlled? 15:00 WORKSHOP 3 - FEEDBACK 15:15 BREAK 15:30 REGULATORY COMPLIANCE GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 PANEL SESSION An opportunity for delegates to put questions to the panel of speakers. 16:45 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Learn

AVAILABLE ONLY TO APPLICANTS WHO HAVE ENROLLED ON (OR HAVE PREVIOUSLY COMPLETED) OUR INTRODUCTION TO PHLEBOTOMY COURSE Geopace Certificate of Competency Add live blood draws to your training Follow and complete a Competency Checklist Obtain tutor sign-off for live draws Awards a CPD Accredited Certificate Awards a Geopace Certificate of Competency Half day or evening sessions

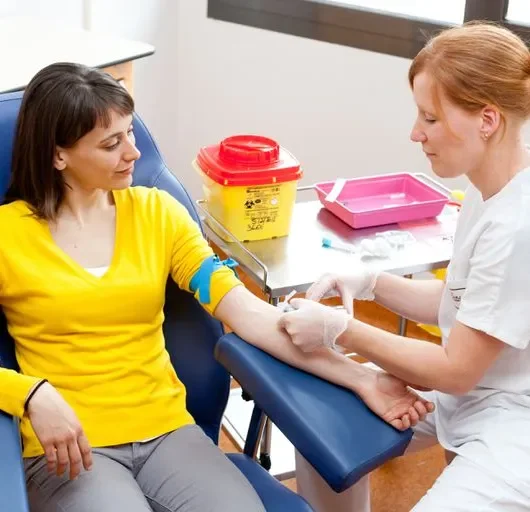
Learn how to take blood ... train as a Phlebotomist Nationally Recognised Qualification No previous experience or qualifications needed OCN Accredited - Level 3 (advanced) CPD Accredited (The CPD Certification Service) Covers all steps up to live blood draw Practise on artificial arm and fake blood! Basic understanding of English language required OPEN TO ALL APPLICANTS

Process Mapping and Standard Operating Procedure (SOP) Writing
By Research Quality Association
COURSE INFORMATION This course aims to empower you with the expertise to proficiently navigate process mapping and master the art of crafting Standard Operating Procedures (SOPs) within regulated environments. Whether you're engaged in activities that demand process improvement, continual enhancement, or SOP creation, this course offers invaluable insights tailored to your needs. It caters to individuals tasked with managing, documenting, and implementing processes and SOPs, irrespective of prior experience or skills. Our curriculum does not focus on specific software or approaches, focusing instead on fundamental principles and adaptable concepts applicable across diverse organisational landscapes. BENEFITS INCLUDE: * Hands-on experience in mapping processes * An understanding of how process maps can be used to define, communicate and continually improve complex processes * An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text * An insight into the preparation of concise and user friendly SOPs. THIS COURSE IS STRUCTURED TO ENCOURAGE DELEGATES TO: * Discuss and develop ideas * Develop a practical approach for creating process maps and writing SOPs * Understand how to use process maps in SOPs effectively * Discuss how process mapping for preparing SOPs can be applied to process improvement for SOPs. IS THIS COURSE FOR YOU? The course is designed for all those with responsibility for managing, documenting and implementing processes and SOPs. It assumes no prior experience or skills. The course does not recommend any specific software or approach, but explores the principles and ideas that can be applied in any organisation. WHAT WILL YOU LEARN? * Hands-on experience in mapping processes * An understanding of how process maps can be used to define, communicate and continually improve complex processes * An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text * An insight into the preparation of concise and user friendly SOPs. By the end of the course you will be able to: * Create process maps and write clearer more concise SOPs * Understand how to use process maps in SOPs effectively * Understand how process mapping can be applied to process improvement and better SOPs. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): LAURA BROWN Director, Laura Brown Training and Development DAVID BUTLER VP of Quality, Resolian -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 09:00 WELCOME, INTRODUCTIONS AND COURSE OBJECTIVES Why Companies manage by process. 10:00 EXERCISE 1 - FIRST STEPS Mechanisms for process mapping, levels at which it can be applied, and the roles and responsibilities of process owners. 10:30 EXERCISE 1 - FEEDBACK 10:45 BREAK 11:00 MAPPING PROCESSES The stages involved in mapping processes including how to construct a SIPOC chart. 11:45 EXERCISE 2 - MAPPING A PROCESS A first opportunity to practice your new process mapping skills. 12:30 EXERCISE 2 - FEEDBACK 13:00 LUNCH 14:00 EXERCISE 3 - DISCUSSION OF OPTIONS FOR FORMAT, STRUCTURE AND LAYOUT What Makes A Good SOP? Discussion of options for format, structure, level of detail of SOPs and the use of process maps for SOP writing. A case study example of a good SOP which uses a flow chart/process map. 14:45 EXERCISE 3 - FEEDBACK 15:00 BREAK 15:15 A CASE STUDY EXAMPLE OF A GOOD SOP THAT USES A FLOW CHART / PROCESS MAP 15:30 EXERCISE 4 Discussion of the content of an SOP using a Process Map 15:35 EXERCISE 4 - FEEDBACK 15:45 KEY WRITING CONSIDERATIONS 16:15 EXERCISE 5 16:45 EXERCISE 5 - FEEDBACK 17:00 CLOSE OF DAY DAY 2 09:00 REVIEW OF DAY 1 09:15 USING METRICS Selecting and using metrics to monitor and improve processes. 10:00 EXERCISE 6 - PRACTICE IN PROCESS MAPPING Creating Process Maps from multi-source information. 11:00 BREAK 11:15 EXERCISE 6 - FEEDBACK 11:45 COMMON PITFALLS Common problems and tips for good Process Mapping. 12:15 DEMONSTRATION OF PROCESS MAPPING ON A PC 12:45 LUNCH 13:30 EXERCISE 7 - MIND MAPPING 14:30 EXERCISE 7 - FEEDBACK 15:00 BREAK 15:15 EXERCISE 8 - USING PROCESS MAPS TO WRITE AN SOP Applying your process mapping skills as part of writing an SOP 15:30 EXERCISE 8 - FEEDBACK 15:45 COURSE REVIEW AND FOLLOW-UP 16:00 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. DEVELOPMENT LEVEL Learn

Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
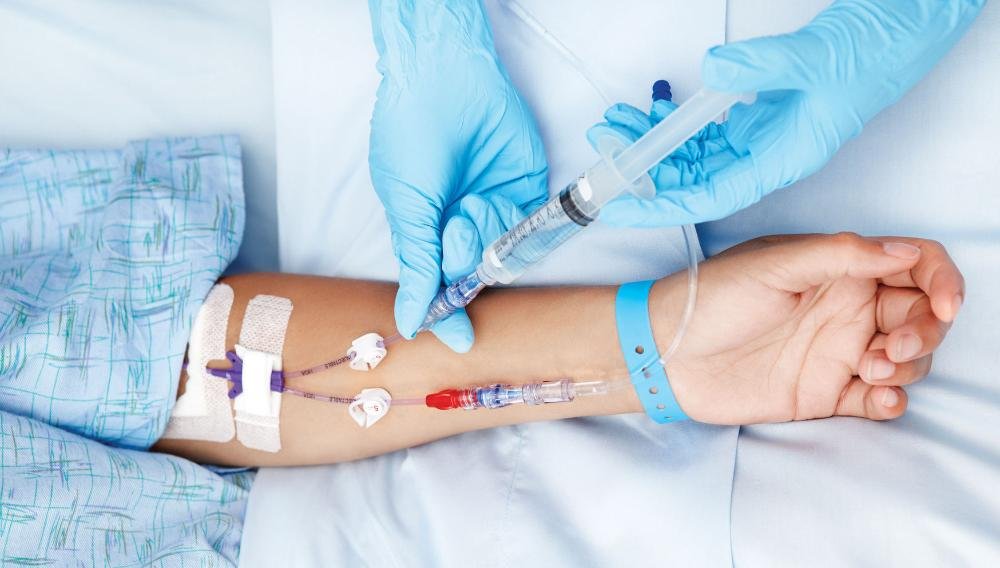
Implementing Good Clinical Laboratory Practice
By Research Quality Association
COURSE INFORMATION Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. IS THIS COURSE FOR YOU? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. THIS COURSE WILL GIVE YOU: * Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) * Insight into the seamless integration of GCLP within clinical programmes (GCP) * Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories * The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) * Access to a seasoned panel of speakers with extensive expertise * A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. ENGAGE IN: * Lively discussions to foster ideas * Problem-solving sessions targeting specific challenges * Detailed exploration of specific aspects within the realms of GCP and GCLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): VANESSA GRANT -, - LOUISE HANDY Director, Handy Consulting Ltd TIM STILES Consultant, Qualogy Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:50 REGISTRATION 09:00 WELCOME AND INTRODUCTION 09:20 GOOD CLINICAL PRACTICE AND THE REQUIREMENTS OF GOOD CLINICAL LABORATORY PRACTICE A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 SAFETY AND ETHICAL CONSIDERATION Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 BREAK 10:55 ORGANISATION AND PERSONNEL RESPONSIBILITIES WITHIN GCP AND THE LABORATORY The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 STAFF TRAINING AND TRAINING RECORDS Personnel records of training and competency assessments are discussed. 11:45 LABORATORY FACILITIES, EQUIPMENT AND MATERIALS Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 LUNCH 13:15 WORKSHOP 1 - FACILITIES, EQUIPMENT AND RESPONSIBILITIES Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 WORKSHOP 1 - FEEDBACK 14:15 COMPUTER SYSTEMS VALIDATION Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 TRIAL PROTOCOLS, ANALYTICAL PLANS During this session we examine the purpose, content, control and change of these important documents. 15:30 BREAK 15:45 WORKSHOP 2 - SOPS, CLINICAL PROTOCOLS, ANALYTICAL PLANS AND VALIDATION The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 WORKSHOP 2 - FEEDBACK 17:00 CLOSE OF DAY DAY 2 09:00 CONDUCT OF THE WORK AND QUALITY CONTROL Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 DEVIATION MANAGEMENT The expectations around deviations and CAPA are discussed. 10:15 WORKSHOP 3 - CONDUCT OF THE WORK AND QUALITY CONTROL Practical work conduct and quality control issues are explored. 10:45 BREAK 11:00 WORKSHOP 3 - FEEDBACK 11:30 SOURCE DATA, DATA INTEGRITY, RECORDS AND REPORTS The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 WORKSHOP 4 - DATA, RECORDS AND REPORTS Practical problems with data, records and reports are investigated. 12:45 LUNCH 13:30 WORKSHOP 4 - FEEDBACK 14:00 QUALITY AUDIT The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 RISK MANAGEMENT How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 BREAK 15:30 REGULATORY INSPECTION The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 PANEL SESSION This panel session will address any outstanding issues raised by the delegates. 16:15 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Develop

Educators matching "Medicine"
Show all 56Search By Location
- Medicine Courses in London
- Medicine Courses in Birmingham
- Medicine Courses in Glasgow
- Medicine Courses in Liverpool
- Medicine Courses in Bristol
- Medicine Courses in Manchester
- Medicine Courses in Sheffield
- Medicine Courses in Leeds
- Medicine Courses in Edinburgh
- Medicine Courses in Leicester
- Medicine Courses in Coventry
- Medicine Courses in Bradford
- Medicine Courses in Cardiff
- Medicine Courses in Belfast
- Medicine Courses in Nottingham








