- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
799 Manufacturing courses delivered Online
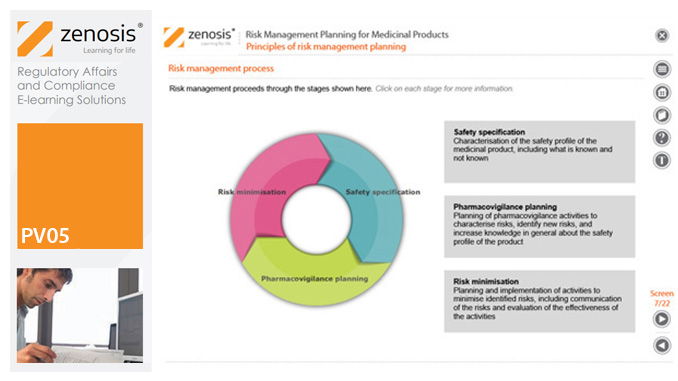
PV05: Risk Management Planning for Medicinal Products
By Zenosis
Proactive risk management is a major component of good pharmacovigilance practice. This module sets out the principles of risk management planning and outlines regulatory requirements for risk management plans in regions that are major markets for medicinal products.
SUB16: The 505(b)(2) Application for Marketing Approval in the USA
By Zenosis
A 505(b)(2) New Drug Application (NDA) is a submission to the Food and Drug Administration (FDA) for approval to market a drug in the USA. It differs from a ‘stand-alone’ NDA in that some of the data on which the applicant relies to demonstrate safety and efficacy have been obtained from publicly available sources rather than from the applicant’s own studies. The applicant typically proposes to market a drug that is based on an approved reference product but modified in its formulation or uses. A 505(b)(2) NDA also differs from an Abbreviated New Drug Application (ANDA) for approval of a generic drug in that the applicant’s product need not be a duplicate of the reference listed drug. The 505(b)(2) pathway may be said to lie part-way between the ‘stand-alone’ NDA and generics pathways, offering a unique combination of advantages to developers. It facilitates the modification of drugs to address unmet medical needs. The 505(b)(2) application pathway accounts for about half of all new drug approvals in the USA.

MD01: An Introduction to the Regulation of Medical Devices
By Zenosis
This module provides an introduction to the basics of medical device regulation, especially the requirements that manufacturers must meet in order to market devices in Europe and the USA.

CT12: How to Conduct Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course describes the requirements that must be met by, and options available to, the sponsor during the conduct of an authorised clinical trial. It identifies the various interactions with MSCs that occur via the Clinical Trials Information System (CTIS), and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT11 sets out the European legal and regulatory context for clinical trials and describes how to apply via the CTIS for authorisation to conduct trials. The two courses therefore provide an ideal foundation for understanding and complying with the new law.

ESS02: Essentials of Monoclonal Antibodies
By Zenosis
This module will introduce you to monoclonal antibodies, explaining how they work, how they are made, and the many uses to which they are put.

PV07: Good Pharmacoepidemiology Practice
By Zenosis
Pharmacoepidemiology is the study of the use and effects of drugs in large numbers of people. It provides a bridge between clinical pharmacology and epidemiology. The increasing demand for real-world evidence of the safety, efficacy and utility of medicinal products has focused greater attention on pharmacoepidemiological research. This module will help those who plan and conduct such research, and analyse and report the findings, to follow good practice.

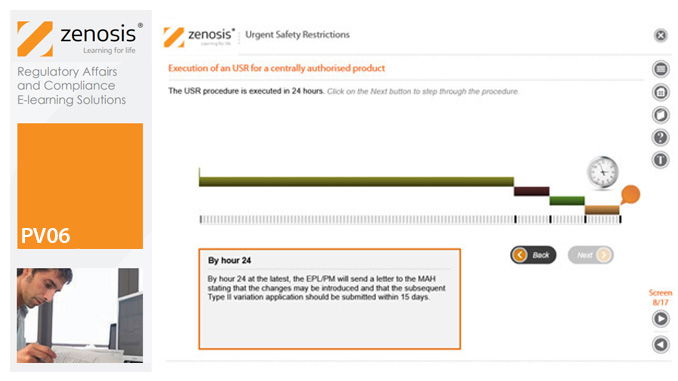
PV06: Urgent Safety Restrictions
By Zenosis
An Urgent Safety Restriction (USR) is a regulatory action taken, in response to a safety signal, to make an interim change to the terms of the marketing authorisation for a medicinal product in Europe. This module describes the principles and procedures for USRs.
Master Abrasive Wheel Safety with our expert training course. Learn about wheel types, safe operations, and inspection techniques to ensure workplace safety and compliance. Perfect for professionals in manufacturing, engineering, and construction sectors.


