- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
61299 Courses delivered Online
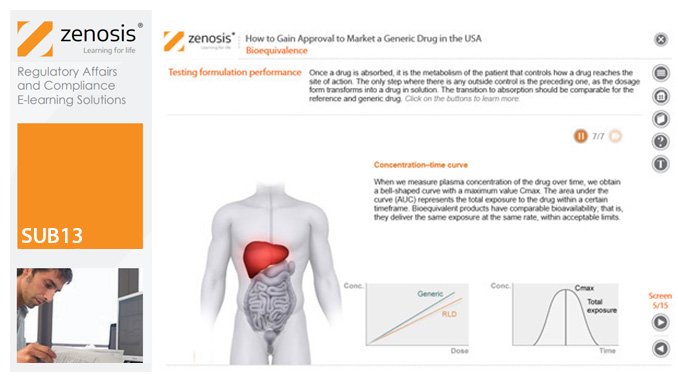
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
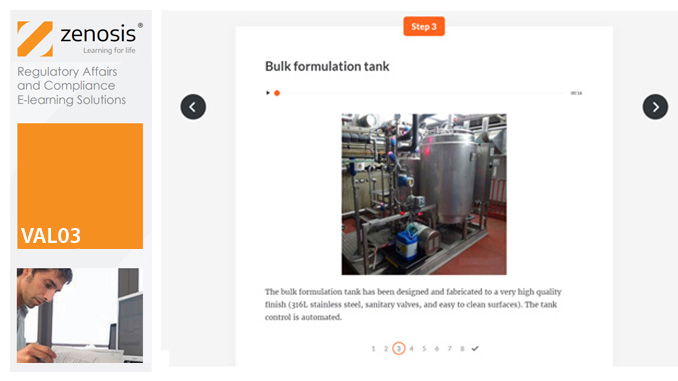
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
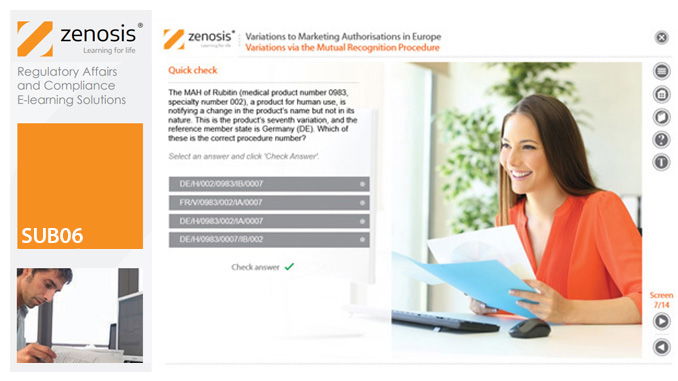
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
🚀 Introduction to Coaching Course: Unlock Your Coaching Potential! 🌟 Discover the art of coaching with our CPD Accredited 'Introduction to Coaching' course. 🎓 Gain insights into the GROW Model, motivation techniques, and advanced questioning skills. Ideal for aspiring coaches and professionals seeking career development.

Risk Management & Outsourcing School Excursions, Camps & Activities
By Xcursion
Risk Management & Outsourcing School Excursions, Camps & Activities Do you contract out school camps, excursions or international tours? If so, you need to understand what risks you've transferred & what risks you've retained. This course steps you through exactly what you need to know when contracting something out.

Investment - QLS Level 2 & 3
By Imperial Academy
Level 3 QLS Endorsed Course | Endorsed Certificate Included | Plus 5 Career Guided Courses | CPD Accredited

Office Management Training
By Imperial Academy
Level 3 Diploma(FREE QLS Endorsed Certificate)| 11 CPD Courses+11 PDF Certificates| 150 CPD Points|CPD & CiQ Accredited

Construction Cost Estimation
By Imperial Academy
Level 5 Diploma (FREE QLS Endorsed Certificate)| 11 CPD Courses+11 PDF Certificate| 145 CPD Points| CPD & CiQ Accredited

