- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
61294 Courses delivered Online
Building Surveying Level 5 - QLS Endorsed Course
By Training Express

Weight Loss Accountability
By Weight Loss Accountability

Site Management Safety Training (SMSTS) Course
By Training Express

Diploma of Counselling and Psychology (Online) CPD Certified Course
By Training Express

SEN Teaching Assistant Level 3 Certificate - QLS Endorsed Course
By Training Express

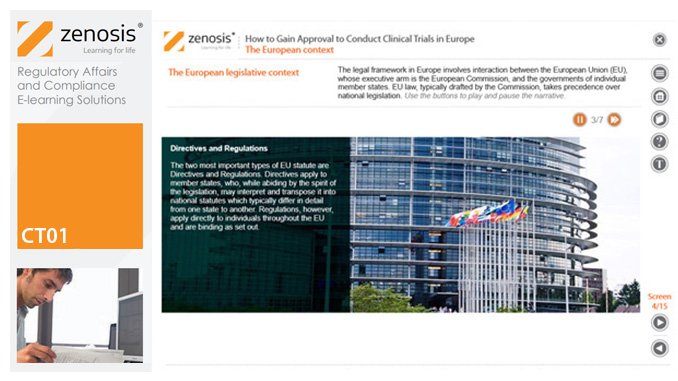
CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
By Zenosis
To conduct a clinical trial in the European Economic Area under the Clinical Trials Directive the sponsor must apply for authorisation from the national competent authority (i.e. medicines regulator), and favourable opinion must be obtained from a research ethics committee, in each member state in which the trial is to take place. This module sets out the requirements for successful compilation, submission and maintenance of the applications.
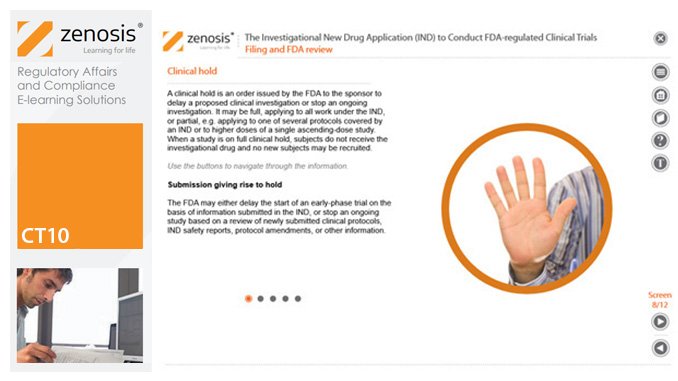
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.


