- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Acupressure for Pain Management : Effective Strategies for Natural Relief
5.0(2)By Institute of Beauty & Makeup
11 in 1 Exclusive Bundle! CPD Certified | Audio Visual Training | 110 CPD Points | Free Certificates | Lifetime Access

Global Healthcare Management - Online Course
By One Education

Professional Certificate Course in Sociowork in Communities Individuals and Families in London 2024
4.9(261)By Metropolitan School of Business & Management UK

Lab Technician at QLS Level 7 Diploma
By Imperial Academy
Level 7 QLS Endorsed Diploma | 11-in-1 Bundle | CPD Accredited | Lifetime Access | Tutor Support

DNA and Genealogy Course
By Apex Learning

PRP / PRF SKIN & HAIR COURSE
By Harley Elite Academy (HeLa)
Hospital Receptionist & Secretary
By Training Tale

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

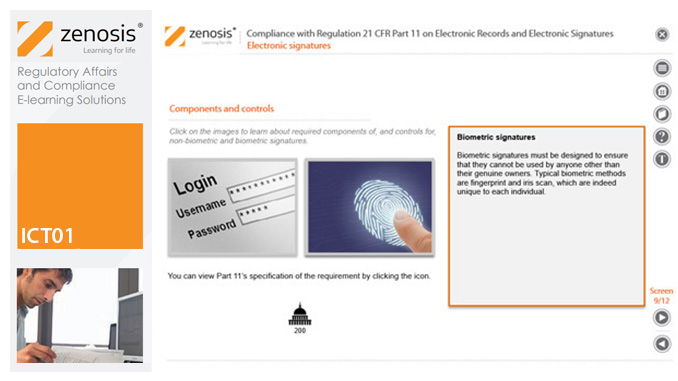
ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.