Clinical Research Associate (CRA) courses in Cardiff
We couldn't find any listings for your search. Explore our online options below.
Know someone teaching this? Help them become an Educator on Cademy.
Get a 10% discount on your first order when you use this promo code at checkout: MAY24BAN3X
Online Options
Show all 63***24 Hour Limited Time Flash Sale*** Clinical Research Associate (CRA) Diploma Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter The UK clinical research market is booming, expected to reach a staggering £27.5 billion by 2025! Are you ready to play your part in developing life-saving treatments and shaping the future of healthcare? If so, this Clinical Research Associate Diploma could be your gateway to a rewarding career. This Clinical Research Associate Diploma empowers you to become a Clinical Research Associate, playing a vital role in developing new drugs and treatments. You'll master clinical research administration, understand scientific concepts, and develop communication, data management, and project management skills. Gain industry-recognized credentials and launch your career in this rewarding and impactful field. But that's not all. When you enrol in Clinical Research Associate Diploma Online Training, you'll receive 25 CPD-Accredited PDF Certificates, Hard Copy Certificates, and our exclusive student ID card, all absolutely free. Courses Are Included In this Clinical Research Associate Diploma Career Bundle: * Course 01: Clinical Research Administration: Navigating the Healthcare Landscape * Course 02: Research in Adult Care * Course 03: Introduction to Medical Terminology * Course 04: Anatomy and Physiology of the Human Body * Course 05: Introduction to Health Economics and Health Technology Assessment * Course 06: Clinical Governance Level 5 (endorsed by The Quality Licence Scheme) * Course 07: Diploma in Clinical Coding (CPD Accredited) * Course 08: Medical Terminology: * Course 09: Biochemistry * Course 10: Genetics * Course 11: Neurology * Course 12: Pharmacology * Course 13: Pharmacy Assistant and Technician Foundation Diploma * Course 14: Mental Health and Psychiatry Training * Course 15: Infection Prevention and Immunisation * Course 16: Healthcare GDPR Training * Course 17: Medical Secretary Diploma * Course 18: Medical Receptionist Course - CPD Accredited * Course 19: Medical Law * Course 20: Medical Transcription: Audio Typist * Course 21: Nurse Prescribing Diploma * Course 22: Radiography * Course 23: Strengthen Your Immune System * Course 24: Medical Writing * Course 25: Effective Communication Techniques for Teachers and Trainers * Course 26: Public Health * Course 27: Initial Training of Community Health Agents * Course 28: Risk Assessment in Health & Social Care With Clinical Research Associate Diploma, you'll embark on an immersive learning experience that combines interactive lessons with voice-over audio, ensuring that you can learn from anywhere in the world, at your own pace. And with 24/7 tutor support, you'll never feel alone in your journey, whether you're a seasoned professional or a beginner. Don't let this opportunity pass you by. Enrol now in Clinical Research Associate Diploma today and take the first step towards achieving your goals and dreams. Why buy this Clinical Research Associate Diploma? * Free CPD Accredited Certificate upon completion of Clinical Research Associate Diploma * Get a free student ID card with a Clinical Research Associate Diploma * Lifetime access to the Clinical Research Associate Diploma course materials * Get instant access to this Clinical Research Associate Diploma course * Learn Clinical Research Associate Diploma from anywhere in the world * 24/7 tutor support with the Clinical Research Associate Diploma course. Start your learning journey straightaway with our Clinical Research Associate Diploma Training! Clinical Research Associate Diploma premium bundle consists of 28 precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of Clinical Research Associate Diploma is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Certification of Clinical Research Associate Diploma You have to complete the assignment given at the end of the Clinical Research Associate Diploma course. After passing the Clinical Research Associate Diploma exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 250 CPD hours / points Accredited by CPD Quality Standards WHO IS THIS COURSE FOR? This Clinical Research Associate Diploma course is ideal for: * Students seeking mastery in Clinical Research Associate * Professionals seeking to enhance Clinical Research Associate skills * Individuals looking for a Clinical Research Associate related career. * Anyone passionate about the Clinical Research Associate REQUIREMENTS This Clinical Research Associate Diploma doesn't require prior experience and is suitable for diverse learners. CAREER PATH * Clinical Research Coordinator (CRC): £26,000 - £34,000 * Clinical Trial Assistant (CTA): £25,000 - £30,000 * Data Manager: £35,000 - £45,000 * Regulatory Affairs Specialist: £40,000 - £55,000 * Medical Writer: £42,000 - £58,000 * Clinical Project Manager: £45,000 - £65,000 CERTIFICATES CPD ACCREDITED DIGITAL CERTIFICATE Digital certificate - Included CPD ACCREDITED HARD COPY CERTIFICATE Hard copy certificate - Included If you are an international student, then you have to pay an additional 10 GBP for each certificate as an international delivery charge.

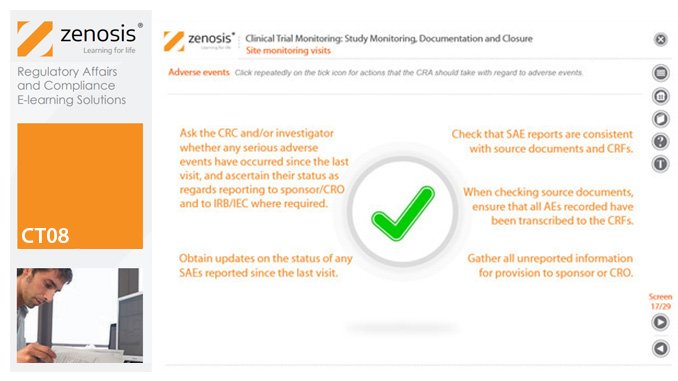
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
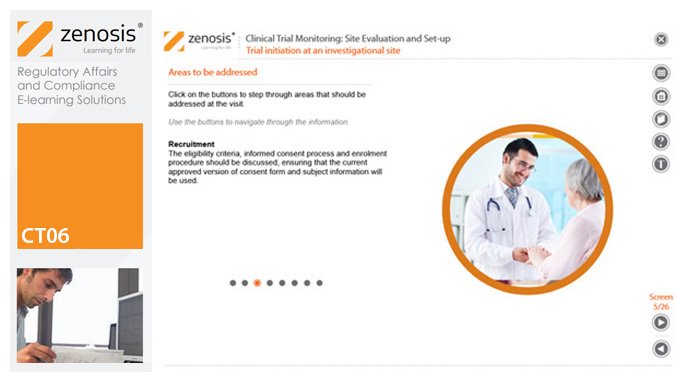
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
Explore the intricacies of Clinical Research Administration with our comprehensive course on Protocols and Ethics. Gain expertise in trial design, ethical considerations, regulatory compliance, and more. Prepare for a future in clinical research with practical insights and knowledge. Enroll now for a rewarding journey in advancing healthcare through responsible and ethical clinical trials.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
COURSE INFORMATION Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. IS THIS COURSE FOR YOU? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. THIS COURSE WILL GIVE YOU: * Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) * Insight into the seamless integration of GCLP within clinical programmes (GCP) * Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories * The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) * Access to a seasoned panel of speakers with extensive expertise * A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. ENGAGE IN: * Lively discussions to foster ideas * Problem-solving sessions targeting specific challenges * Detailed exploration of specific aspects within the realms of GCP and GCLP. -------------------------------------------------------------------------------- TUTORS Tutors will be comprised of (click the photos for biographies): VANESSA GRANT -, - LOUISE HANDY Director, Handy Consulting Ltd TIM STILES Consultant, Qualogy Ltd -------------------------------------------------------------------------------- PROGRAMME Please note timings may be subject to alteration. DAY 1 08:50 REGISTRATION 09:00 WELCOME AND INTRODUCTION 09:20 GOOD CLINICAL PRACTICE AND THE REQUIREMENTS OF GOOD CLINICAL LABORATORY PRACTICE A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 SAFETY AND ETHICAL CONSIDERATION Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 BREAK 10:55 ORGANISATION AND PERSONNEL RESPONSIBILITIES WITHIN GCP AND THE LABORATORY The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 STAFF TRAINING AND TRAINING RECORDS Personnel records of training and competency assessments are discussed. 11:45 LABORATORY FACILITIES, EQUIPMENT AND MATERIALS Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 LUNCH 13:15 WORKSHOP 1 - FACILITIES, EQUIPMENT AND RESPONSIBILITIES Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 WORKSHOP 1 - FEEDBACK 14:15 COMPUTER SYSTEMS VALIDATION Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 TRIAL PROTOCOLS, ANALYTICAL PLANS During this session we examine the purpose, content, control and change of these important documents. 15:30 BREAK 15:45 WORKSHOP 2 - SOPS, CLINICAL PROTOCOLS, ANALYTICAL PLANS AND VALIDATION The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 WORKSHOP 2 - FEEDBACK 17:00 CLOSE OF DAY DAY 2 09:00 CONDUCT OF THE WORK AND QUALITY CONTROL Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 DEVIATION MANAGEMENT The expectations around deviations and CAPA are discussed. 10:15 WORKSHOP 3 - CONDUCT OF THE WORK AND QUALITY CONTROL Practical work conduct and quality control issues are explored. 10:45 BREAK 11:00 WORKSHOP 3 - FEEDBACK 11:30 SOURCE DATA, DATA INTEGRITY, RECORDS AND REPORTS The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 WORKSHOP 4 - DATA, RECORDS AND REPORTS Practical problems with data, records and reports are investigated. 12:45 LUNCH 13:30 WORKSHOP 4 - FEEDBACK 14:00 QUALITY AUDIT The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 RISK MANAGEMENT How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 BREAK 15:30 REGULATORY INSPECTION The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 PANEL SESSION This panel session will address any outstanding issues raised by the delegates. 16:15 CLOSE OF COURSE -------------------------------------------------------------------------------- EXTRA INFORMATION Face-to-face course COURSE MATERIAL Course material will be available in PDF format for delegates attending this course. The advantages of this include: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course COURSE MATERIAL This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. THE ADVANTAGES OF THIS INCLUDE: * Ability for delegates to keep material on a mobile device * Ability to review material at any time pre and post course * Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD POINTS 14 Points DEVELOPMENT LEVEL Develop

***24 Hour Limited Time Flash Sale*** Clinical Research Associate Diploma - CPD Certified Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Are you a professional feeling stuck in your career, struggling to keep up with the ever-changing demands of the industry? Or perhaps you're a beginner, unsure of where to start or how to break into your desired field. Whichever stage you're in, our exclusive Clinical Research Associate Diploma - CPD Certified Bundle provides unique insights and tools that can help you achieve your goals. Designed to cater to the needs of both seasoned professionals and aspiring newcomers, our Clinical Research Associate Diploma - CPD Certified bundle is a comprehensive program that will equip you with the essential skills and knowledge you need to succeed. Whether you're looking to advance in your current role or embark on a new career journey, this bundle has everything you need to take your professional life to the next level. But that's not all. When you enrol in Clinical Research Associate Diploma - CPD Certified Online Training, you'll receive 25 CPD-Accredited PDF Certificates, Hard Copy Certificates, and our exclusive student ID card, all absolutely free. Courses Are Included In this Clinical Research Associate Diploma - CPD Certified Career Bundle: * Course 01: Clinical Research Administration: Navigating the Healthcare Landscape * Course 02: Research in Adult Care * Course 03: Introduction to Medical Terminology * Course 04: Anatomy and Physiology of Human Body * Course 05: Introduction to Health Economics and Health Technology Assessment * Course 06: Biochemistry * Course 07: Genetics * Course 08: Neurology * Course 09: Pharmacology * Course 10: Pharmacy Assistant and Technician Foundation Diploma * Course 11: Mental Health and Psychiatry Training * Course 12: Infection Prevention and Immunisation * Course 13: Healthcare GDPR Training * Course 14: Medical Secretary Diploma * Course 15: Medical Receptionist Course - CPD Accredited * Course 16: Medical Law * Course 17: Medical Transcription: Audio Typist * Course 18: Nurse Prescribing Diploma * Course 19: Radiography * Course 20: Strengthen Your Immune System * Course 21: Medical Writing * Course 22: Effective Communication Techniques for Teachers and Trainers * Course 23: Public Health * Course 24: Initial Training of Community Health Agents * Course 25: Risk Assessment in Health & Social Care With Level 7 in Strategic Learning & Development, you'll embark on an immersive learning experience that combines interactive lessons with voice-over audio, ensuring that you can learn from anywhere in the world, at your own pace. And with 24/7 tutor support, you'll never feel alone in your journey, whether you're a seasoned professional or a beginner. Don't let this opportunity pass you by. Enrol in Clinical Research Associate Diploma - CPD Certified today and take the first step towards achieving your goals and dreams. Why buy this Level 7 in Strategic Learning & Development? * Free CPD Accredited Certificate upon completion of Level 7 in Strategic Learning & Development * Get a free student ID card with Level 7 in Strategic Learning & Development * Lifetime access to the Clinical Research Associate Diploma - CPD Certified course materials * Get instant access to this Clinical Research Associate Diploma - CPD Certified course * Learn Clinical Research Associate Diploma - CPD Certified from anywhere in the world * 24/7 tutor support with the Clinical Research Associate Diploma - CPD Certified course. Start your learning journey straightaway with our Clinical Research Associate Diploma - CPD Certified Training! Clinical Research Associate Diploma - CPD Certified premium bundle consists of 25 precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of the Clinical Research Associate Diploma - CPD Certified is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Certification You have to complete the assignment given at the end of the Clinical Research Associate Diploma - CPD Certified course. After passing the Clinical Research Associate Diploma - CPD Certified exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 250 CPD hours / points Accredited by CPD Quality Standards WHO IS THIS COURSE FOR? This Clinical Research Associate Diploma - CPD Certified course is ideal for: * Students seeking mastery in Level 7 in Strategic Learning & Development * Professionals seeking to enhance Clinical Research Associate Diploma - CPD Certified skills * Individuals looking for a Level 7 in Strategic Learning & Development-related career. * Anyone passionate about Level 7 in Strategic Learning & Development REQUIREMENTS This Clinical Research Associate Diploma - CPD Certified doesn't require prior experience and is suitable for diverse learners. CAREER PATH This Clinical Research Associate Diploma - CPD Certified bundle will allow you to kickstart or take your career in the related sector to the next stage. CERTIFICATES CPD ACCREDITED HARD COPY CERTIFICATE Hard copy certificate - Included If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate CPD ACCREDITED DIGITAL CERTIFICATE Digital certificate - Included

OVERVIEW Explore the intricacies of clinical research and understand the administration process with our Clinical Research Administration Training course. This course will help you deepen your knowledge and acquire the ability to seek career prospects in the relevant sectors. The course is packed with high;y valuable information on clinical research. From the informative modules, you will learn the core concepts of clinic trial designing and planning. The course will help you develop the skills for data management and analysis. You will be able to build your expertise in monitoring and auditing. Moreover, you will acquire the skills required for quality control and site management as well. After the course, you will receive a CPDQS-accredated certificate of achievement. This certificate will enhance your employability and help you reach your career goals. Enrol now! COURSE PREVIEW LEARNING OUTCOMES * Understand the significance of clinical research in healthcare * Learn about the process of clinical trail design and planning * Deepen your knowledge of ethics and regulatory compliance * Develop the ability for data management and record-keeping * Know how to monitor and audit the trials * Grasp the process of study site management and quality control WHY TAKE THIS COURSE FROM JOHN ACADEMY? * Affordable, well-structured and high-quality e-learning study materials * Engaging tutorial videos, materials from the industry-leading experts * Opportunity to study in a user-friendly, advanced online learning platform * Efficient exam systems for the assessment and instant result * Earn UK & internationally recognised accredited qualification * Easily access the course content on mobile, tablet, or desktop from anywhere, anytime * Excellent career advancement opportunities * Get 24/7 student support via email. WHAT SKILLS WILL YOU LEARN FROM THIS COURSE? * Trail planning * Data management and analysis * Monitoring and auditing * Site management * Quality control * Compliance management WHO SHOULD TAKE THIS CLINICAL RESEARCH ADMINISTRATION TRAINING? Whether you're an existing practitioner or an aspiring professional, this course is an ideal training opportunity. It will elevate your expertise and boost your CV with key skills and a recognised qualification attesting to your knowledge. ARE THERE ANY ENTRY REQUIREMENTS? This Clinical Research Administration Training course is available to all learners of all academic backgrounds. But learners should be aged 16 or over to undertake the qualification. And a good understanding of the English language, numeracy, and ICT will be helpful. CERTIFICATE OF ACHIEVEMENT After completing this course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates & Transcripts can be obtained either in Hardcopy at £14.99 or in PDF format at £11.99. CAREER PATHÂ This exclusive Clinical Research Administration Training course will equip you with effective skills and abilities and help you explore career paths such as * Clinical Research Administrator * Clinical Research Associate * Clinical Research Coordinator * Clinical Data Manager Module 01: Introduction to Clinical Research Administration Introduction to Clinical Research Administration 00:15:00 Module 02: Clinical Trial Design and Planning Clinical Trial Design and Planning 00:11:00 Module 03: Ethics and Regulatory Compliance Ethics and Regulatory Compliance 00:13:00 Module 04: Institutional Review Boards (IRBs) and Ethics Committees Institutional Review Boards (IRBs) and Ethics Committees 00:10:00 Module 05: Data Management and Recordkeeping Data Management and Recordkeeping 00:12:00 Module 06: Safety Reporting and Adverse Events Safety Reporting and Adverse Events 00:10:00 Module 07: Clinical Trial Monitoring and Auditing Clinical Trial Monitoring and Auditing 00:09:00 Module 08: Study Site Management and Quality Control Study Site Management and Quality Control 00:17:00 Module 09: Data Analysis and Reporting Data Analysis and Reporting 00:09:00 Module 10: The Future of Clinical Research The Future of Clinical Research 00:10:00 Certificate and Transcript Order Your Certificates and Transcripts 00:00:00

***24 Hour Limited Time Flash Sale*** Clinical Trial Administrator (CTA) Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Are you a professional feeling stuck in your career, struggling to keep up with the ever-changing demands of the industry? Or perhaps you're a beginner, unsure of where to start or how to break into your desired field. Whichever stage you're in, our exclusive Clinical Trial Administrator (CTA) Bundle provides unique insights and tools that can help you achieve your goals. Designed to cater to the needs of both seasoned professionals and aspiring newcomers, our Clinical Trial Administrator (CTA) bundle is a comprehensive program that will equip you with the essential skills and knowledge you need to succeed. Whether you're looking to advance in your current role or embark on a new career journey, this bundle has everything you need to take your professional life to the next level. But that's not all. When you enrol in Clinical Trial Administrator (CTA) Online Training, you'll receive 30 CPD-Accredited PDF Certificates, Hard Copy Certificates, and our exclusive student ID card, all absolutely free. Courses Are Included In this Clinical Trial Administrator (CTA) Career Bundle: * Course 01: Medical & Clinical Administration Diploma * Course 02: Project Management * Course 03: Agile Project Management * Course 04: Level 5 Diploma in Leadership and Management for Adult Care * Course 05: Diploma in Operations Management * Course 06: Clinical Governance Level 5 (endorsed by The Quality Licence Scheme) * Course 07: Quality Control in Healthcare and Clinical Audit * Course 08: Clinical Observations Skills * Course 09: Clinical Psychology * Course 10: An Introduction to Clinical Anaesthesia * Course 11: Diploma in Clinical Coding (CPD Accredited) * Course 12: Supervision in Adult Care * Course 13: Research in Adult Care * Course 14: Introduction to Medical Terminology * Course 15: Anatomy and Physiology of the Human Body * Course 16: Introduction to Health Economics and Health Technology Assessment * Course 17: Initial Training of Community Health Agents * Course 18: Medical Terminology: * Course 19: Healthcare GDPR Training * Course 20: Medical Secretary Diploma * Course 21: Medical Receptionist Course * Course 22: Medical Law * Course 23: Medical Transcription * Course 24: Nurse Prescribing Diploma * Course 25: Pharmacy Assistant and Technician Foundation Diploma * Course 26: Infection Prevention and Immunisation * Course 27: Decision-Making in High-Stress Situations * Course 28: Public Health * Course 29: Effective Communication * Course 30: Risk Assessment in Health & Social Care With Clinical Trial Administrator (CTA), you'll embark on an immersive learning experience that combines interactive lessons with voice-over audio, ensuring that you can learn from anywhere in the world, at your own pace. And with 24/7 tutor support, you'll never feel alone in your journey, whether you're a seasoned professional or a beginner. Don't let this opportunity pass you by. Enrol in Clinical Trial Administrator (CTA) today and take the first step towards achieving your goals and dreams. Why buy this Clinical Trial Administrator (CTA)? * Free CPD Accredited Certificate upon completion of Clinical Trial Administrator (CTA) * Get a free student ID card with Clinical Trial Administrator (CTA) * Lifetime access to the Clinical Trial Administrator (CTA) course materials * Get instant access to this Clinical Trial Administrator (CTA) course * Learn Clinical Trial Administrator (CTA) from anywhere in the world * 24/7 tutor support with the Clinical Trial Administrator (CTA) course. Start your learning journey straightaway with our Clinical Trial Administrator (CTA) Training! Clinical Trial Administrator (CTA) premium bundle consists of 30 precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of Clinical Trial Administrator (CTA) is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Certification You have to complete the assignment given at the end of the Clinical Trial Administrator (CTA) course. After passing the Clinical Trial Administrator (CTA) exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 300 CPD hours / points Accredited by CPD Quality Standards WHO IS THIS COURSE FOR? This Clinical Trial Administrator (CTA) course is ideal for: * Students seeking mastery in Clinical Trial Administrator (CTA) * Professionals seeking to enhance Clinical Trial Administrator (CTA) skills * Individuals looking for a Clinical Trial Administrator (CTA)-related career. * Anyone passionate about Clinical Trial Administrator (CTA) REQUIREMENTS This Clinical Trial Administrator (CTA) doesn't require prior experience and is suitable for diverse learners. CAREER PATH * Clinical Trial Manager * Clinical Research Coordinator * Clinical Operations Manager * Clinical Project Manager * Director of Clinical Operations CERTIFICATES CPD ACCREDITED DIGITAL CERTIFICATE Digital certificate - Included CPD ACCREDITED HARD COPY CERTIFICATE Hard copy certificate - Included If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate

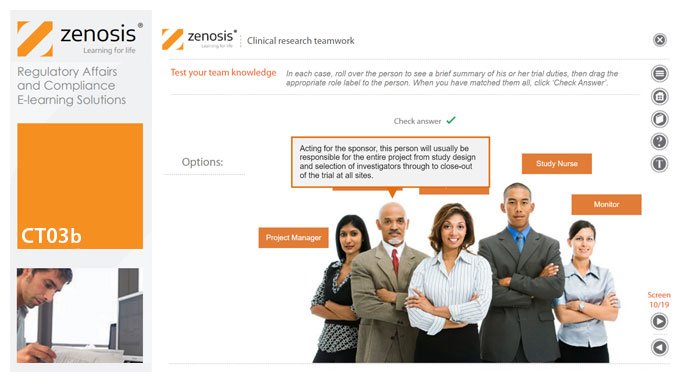
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
Clinical Research Associate: Administration & Navigating the Healthcare Landscape
5.0(2)By Studyhub UK
In the intricate world of healthcare, clinical research is a beacon, shining light on innovations and advancements. Dive into 'Clinical Research Administration: Navigating the Healthcare Landscape', an expertly curated journey that marries in-depth knowledge with actionable strategies. This course will guide you from the foundations of clinical research to its evolving future. You'll traverse trial designs, the imperatives of ethics, data intricacies, and quality controls, ensuring you're equipped to lead, innovate, and make pivotal decisions within the clinical research realm. Learning Outcomes * Gain a robust understanding of the clinical research ecosystem and its administration. * Design and plan effective clinical trials while ensuring ethical and regulatory compliance. * Master the art of data management, ensuring accuracy and reliability. * Oversee trial monitoring, auditing, and meticulous safety reporting. * Analyse clinical data proficiently and forecast the future trends in clinical research. WHY CHOOSE THIS CLINICAL RESEARCH ADMINISTRATION: NAVIGATING THE HEALTHCARE LANDSCAPE COURSE? * Unlimited access to the course for a lifetime. * Opportunity to earn a certificate accredited by the CPD Quality Standards after completing this course. * Structured lesson planning in line with industry standards. * Immerse yourself in innovative and captivating course materials and activities. * Assessments are designed to evaluate advanced cognitive abilities and skill proficiency. * Flexibility to complete the Clinical Research Associate: Administration & Navigating the Healthcare Landscape Course at your own pace, on your own schedule. * Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. WHO IS THIS CLINICAL RESEARCH ADMINISTRATION: NAVIGATING THE HEALTHCARE LANDSCAPE COURSE FOR? * Aspiring clinical researchers poised to shape the future of healthcare. * Healthcare administrators seeking specialised knowledge in research. * Medical students aiming to bolster their understanding of clinical trials. * Ethics committee members desiring a broader perspective. * Quality assurance professionals in the healthcare sector. CAREER PATH * Clinical Research Coordinator: £25,000 - £40,000 * Clinical Data Manager: £35,000 - £60,000 * Clinical Research Associate: £30,000 - £50,000 * Regulatory Affairs Specialist: £40,000 - £70,000 * Clinical Trials Auditor: £40,000 - £65,000 * Clinical Research Manager: £50,000 - £80,000 PREREQUISITES This Clinical Research Associate: Administration & Navigating the Healthcare Landscape does not require you to have any prior qualifications or experience. You can just enrol and start learning.This Clinical Research Associate: Administration & Navigating the Healthcare Landscape was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. CERTIFICATION After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. COURSE CURRICULUM Module 01: Introduction to Clinical Research Administration Introduction to Clinical Research Administration 00:15:00 Module 02: Clinical Trial Design and Planning Ethics and Regulatory Compliance 00:11:00 Module 03: Ethics and Regulatory Compliance Ethics and Regulatory Compliance 00:13:00 Module 04: Institutional Review Boards (IRBs) and Ethics Committees Institutional Review Boards (IRBs) and Ethics Committees 00:10:00 Module 05: Data Management and Recordkeeping Data Management and Recordkeeping 00:12:00 Module 06: Safety Reporting and Adverse Events Safety Reporting and Adverse Events 00:10:00 Module 07: Clinical Trial Monitoring and Auditing Clinical Trial Monitoring and Auditing 00:09:00 Module 08: Study Site Management and Quality Control Study Site Management and Quality Control 00:17:00 Module 09: Data Analysis and Reporting Data Analysis and Reporting 00:09:00 Module 10: The Future of Clinical Research The Future of Clinical Research 00:10:00

Search By Location
- Clinical Research Associate (CRA) Courses in London
- Clinical Research Associate (CRA) Courses in Birmingham
- Clinical Research Associate (CRA) Courses in Glasgow
- Clinical Research Associate (CRA) Courses in Liverpool
- Clinical Research Associate (CRA) Courses in Bristol
- Clinical Research Associate (CRA) Courses in Manchester
- Clinical Research Associate (CRA) Courses in Sheffield
- Clinical Research Associate (CRA) Courses in Leeds
- Clinical Research Associate (CRA) Courses in Edinburgh
- Clinical Research Associate (CRA) Courses in Leicester
- Clinical Research Associate (CRA) Courses in Coventry
- Clinical Research Associate (CRA) Courses in Bradford
- Clinical Research Associate (CRA) Courses in Cardiff
- Clinical Research Associate (CRA) Courses in Belfast
- Clinical Research Associate (CRA) Courses in Nottingham