- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
2324 Courses
Hypnotherapy Learning Retreat, immersive four-day retreat designed to elevate your skills and knowledge in the serene environment of our exclusive venue. Whether you are a seasoned professional or just beginning your journey, this retreat offers a unique opportunity to learn, grow, and connect with like-minded individuals.

Clinical Coding - Online Course
By One Education

Clinical Governance Purpose and Functions
By NextGen Learning

Clinical Observation Skills for Carers Course
By NextGen Learning

Clinical Governance in Adult Care Course
By NextGen Learning

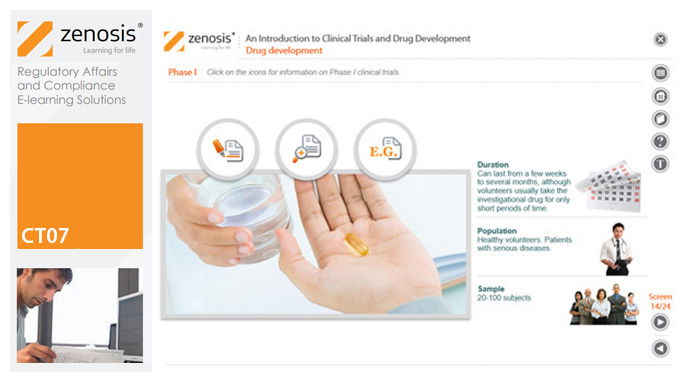
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
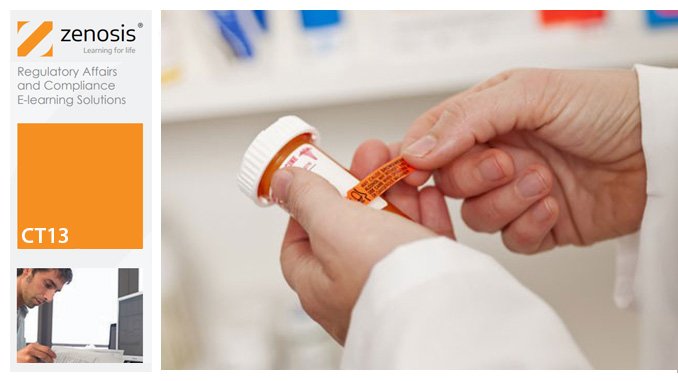
CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
Search By Location
- Clinical Courses in London
- Clinical Courses in Birmingham
- Clinical Courses in Glasgow
- Clinical Courses in Liverpool
- Clinical Courses in Bristol
- Clinical Courses in Manchester
- Clinical Courses in Sheffield
- Clinical Courses in Leeds
- Clinical Courses in Edinburgh
- Clinical Courses in Leicester
- Clinical Courses in Coventry
- Clinical Courses in Bradford
- Clinical Courses in Cardiff
- Clinical Courses in Belfast
- Clinical Courses in Nottingham

