- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
6564 Courses
Health & Social Care, Consent Training and Liberty Safeguards
4.8(12)By Academy for Health and Fitness

Safe Moving and Handling (RQF) Level 2
By BAB Business Group

Business Administration Career Track Diploma Course
By Training Express

Compliance Officer: Understanding Regulatory Compliance Requirements Course
By Training Express

Perhaps you are working on a digital transformation project or AI and whenever this phrase pops up you’re not clear what it means for you. How do you know if you are data literate? Who in your organisation needs to be data literate? Why does it matter? Get up to speed with this 90 minute introductory training which focuses on the key principles of data literacy so you can apply them in your day to day work.

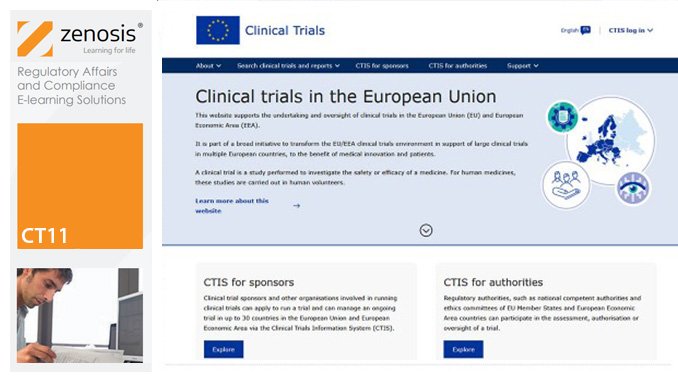
CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
Search By Location
- Law Courses in London
- Law Courses in Birmingham
- Law Courses in Glasgow
- Law Courses in Liverpool
- Law Courses in Bristol
- Law Courses in Manchester
- Law Courses in Sheffield
- Law Courses in Leeds
- Law Courses in Edinburgh
- Law Courses in Leicester
- Law Courses in Coventry
- Law Courses in Bradford
- Law Courses in Cardiff
- Law Courses in Belfast
- Law Courses in Nottingham



