- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
CT04d - Clinical trial endpoints
By Zenosis
In clinical trials, endpoints are measurements to evaluate the results of a new treatment, at an individual patient level. The study data can be extrapolated to patient populations on the basis of clinical similarities to patients participating in the trial. When clinical trial data have been obtained, focus is on the trial endpoints; more specifically, the focus is on whether the trial met or failed the primary endpoint specified before the trial started. The purpose and various types of endpoints are discussed in this short course.
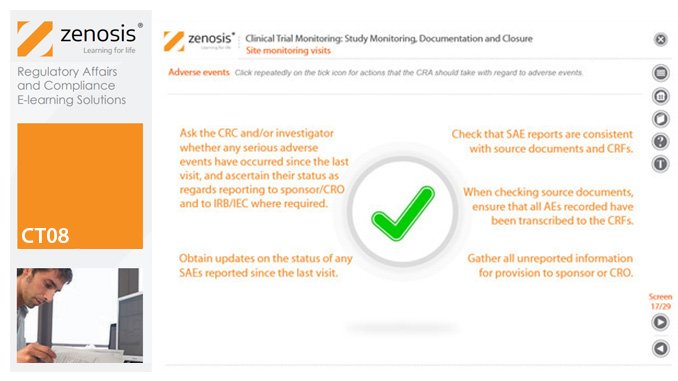
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
Medical & Clinical Administration
By Compliance Central







