- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1693 Courses delivered Online
Digital Functional Skills Entry Level 3 - Online Course and Exam
By Lead Academy
Digital Functional Skills Level 1 - Online Course and Exam
By Lead Academy

AWS Certified Solutions Architect Associate (SAA-C03)
By Packt
Prepare for the AWS Certified Solutions Architect - Associate (SAA-C03) exam. Learn about the AWS Management Console, S3 buckets, instances, database services, cloud security, costs associated with AWS, Amazon Elastic Compute Cloud (EC2), Amazon Virtual Private Cloud (VPC), Amazon Simple Storage Service (S3), and Amazon Elastic Block Store (EBS).

Theory of Constraints, Throughput Accounting and Lean Accounting Level 2
By Course Cloud

Voiceover Artist and Freelance Translation - Endorsed Certificate
By Imperial Academy
Level 5 - Two Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

Surveying- Construction Management and Safety Course
By One Education

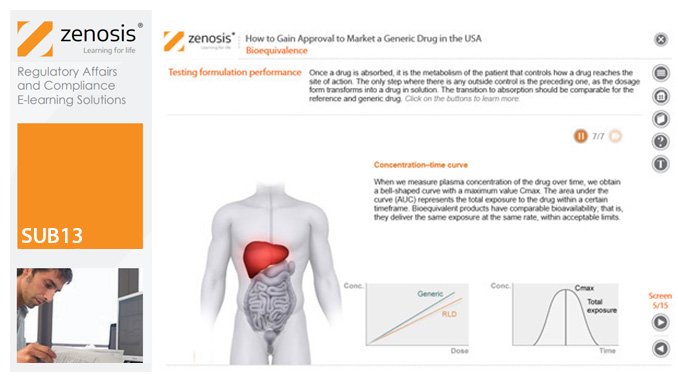
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
Level 7 Diploma in Investment Advisor Certification - QLS Endorsed
4.8(12)By Academy for Health and Fitness

Level 5 Diploma Agile Scrum Master Certification - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

